Figure 3.
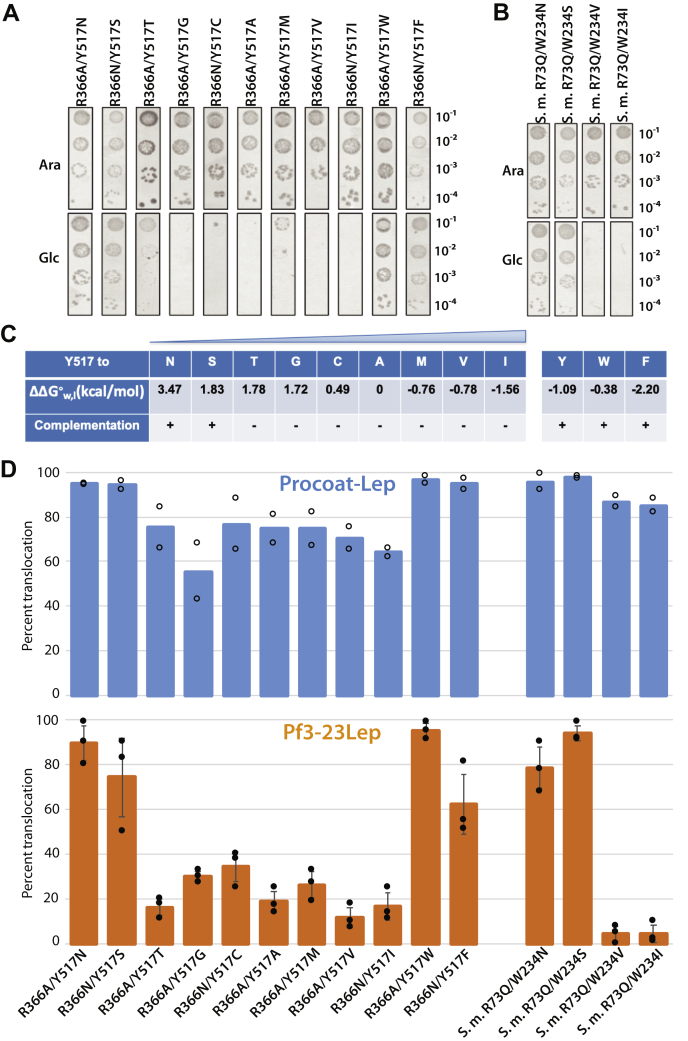
The polarity at the dome of the YidC hydrophilic groove determines the necessity of the conserved positive charge.A, complementation assay to examine the importance of R366 (by mutating R366 to Ala or Asn) for Escherichia coli YidC when Y517 was substituted with Asn, Ser, Gly, Thr, Ala, Cys, Val, Ile, Met, Trp, or Phe. YidC depletion strain JS7131 was transformed with pACYC184 encoding these double mutants and a spot test at 37 °C was performed to test complementation, as described in Figure 2A. Note the data for R366A/Y517A (see Fig. 2A) is included for comparison. B, complementation assay to examine the role of R73 (by substituting R73 with Q) of Streptococcus mutans YidC2 when W234 was mutated to Asn, Ser, Val, or Ile. Note that in S. mutans YidC2, W234 aligns with Y517 in E. coli YidC, while R73 is equivalent to R366. C, summary of complementation results for the E. coli YidC 517 mutants. The hydrophobicity panel shows the standard free energy in kcal/mol for each amino acid tested (42). The “+” indicates that the mutant complemented the YidC depletion strain, indicating the arginine is not essential. The “−” means it did not complement showing the arginine is required for function. D, E. coli YidC and S. mutans 247YidC2 mutants were tested for their ability to insert PC-Lep (blue bars) and Pf3-23Lep (orange bars). Plasmids pACYC184 encoding YidC or YidC2 mutants were cotransformed with pMS119 encoding PC-Lep or Pf3-23Lep into JS7131. After the expression of YidC substrates and labeling, the membrane insertion of PC-Lep and Pf3-23Lep was tested as described in Figure 2B. The results (one representative trial shown in Fig. S2) were quantified as previously described (24) and summarized in panel D. PC, procoat.