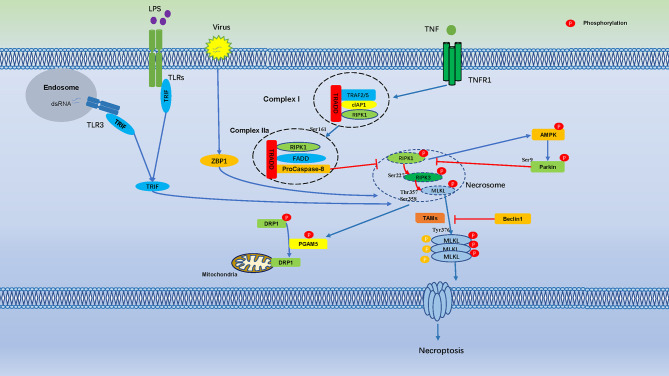
Figure 1.
Cell Necroptosis is Regulated by Numerous Phosphorylation Events. Phosphorylation regulates cell necroptosis through numerous phosphorylation events, TNF bind to TNFR1 on the cell membrane and recruit a series of proteins to form complex I, which is includes TNFR-associated death domain (TRADD), RIPK1, TRAF2, the cellular inhibitor of apoptosis protein 1 (cIAP1). Autophosphorylation of RIPK1 leads to its enzymatic activation, Ser161 of RIPK1 is an important autophosphorylation sites, which triggers RIPK3 phosphorylation, the phosphorylation of Ser227 of RIPK3 triggers MLKL recruitment to the necrosome, which phosphorylates MLKL in Ser358, Thr357, and induces necroptosis. AMPK is activated by RIPKs and subsequently phosphorylates Parkin’s Ser9, which is inhibits phosphorylation of RIPK3. Necrosome promotes phosphorylation of PGAM5, which induces dephosphorylation of DRP1.