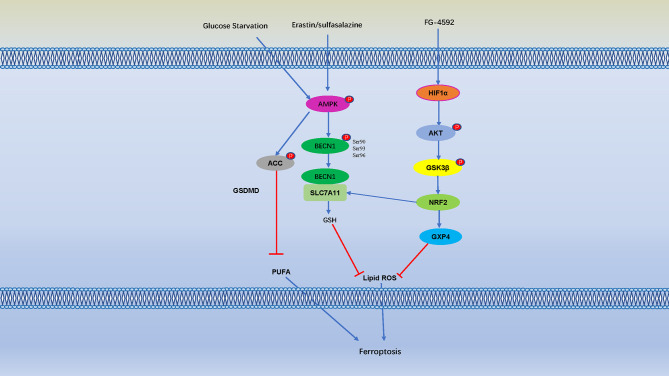
Figure 4.
Cell Ferroptosis is Regulated by Numerous Phosphorylation Events. AMP-activated protein kinase (AMPK) is a key sensor of cellular energy status, and glucose starvation leads to phosphorylation and activation of AMPK, AMPK mediated phosphorylation of Acetyl-CoA carboxylase (ACC) and inhibiting PUFA-containing lipid biosynthesis and negatively regulates ferroptosis. Erastin and sulfasalazine also activate AMPK phosphorylation, and AMPK phosphorylates BECN1 at Ser90/93/96, thereby promoting the formation of the BECN1-SLC7A11 complex (131). The interaction between BECN1 and SLC7A11 promote GSH, thereby inhibit lipid ROS and negatively regulates ferroptosis. Moreover, folic acid induced kidney damage was alleviated by pretreatment of FG-4592, which increased phosphorylated of protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3 β) and activated NFE2-related factor 2 (Nrf2) to inhibit ferroptosis.