Abstract
Primary ventricular fibrillation (PVF) is a major driver of cardiac arrest in the acute phase of ST-segment elevation myocardial infarction (STEMI). Enrichment of cardiomyocyte plasma membranes with dietary polyunsaturated fatty acids (PUFA) reduces vulnerability to PVF experimentally, but clinical data are scarce. PUFA status in serum phospholipids is a valid surrogate biomarker of PUFA status in cardiomyocytes within a wide range of dietary PUFA. In this nested case–control study (n = 58 cases of STEMI-driven PVF, n = 116 control non-PVF STEMI patients matched for age, sex, smoking status, dyslipidemia, diabetes mellitus and hypertension) we determined fatty acids in serum phospholipids by gas-chromatography, and assessed differences between cases and controls, applying the Benjamini–Hochberg procedure on nominal P-values to control the false discovery rate (FDR). Significant differences between cases and controls were restricted to linoleic acid (LA), with PVF patients showing a lower level (nominal P = 0.002; FDR-corrected P = 0.027). In a conditional logistic regression model, each one standard deviation increase in the proportion of LA was related to a 42% lower prevalence of PVF (odds ratio = 0.58; 95% confidence interval, 0.37, 0.90; P = 0.02). The association lasted after the inclusion of confounders. Thus, regular consumption of LA-rich foods (nuts, oils from seeds) may protect against ischemia-driven malignant arrhythmias.
Subject terms: Lipidomics, Cardiology
Introduction
Coronary artery disease (CAD) remains a major health challenge and a top cause of global mortality1. Though advances in drugs and device-based therapies have largely reduced complications and improved outcomes for those who experience a myocardial infarction (MI), the rate of primary ventricular fibrillation (PVF) has remained stable over time2,3. PVF is a major trigger of out-of-hospital cardiac arrest leading to sudden cardiac death4, and patients developing ventricular fibrillation during acute MI are at higher risk of in-hospital mortality5. Therefore, novel strategies are needed to prevent and manage acute ischemic PVF.
Experimental research has been instrumental in understanding the arrhythmogenic mechanisms leading to PVF. In this regard, many metabolic and electrophysiological cardiac changes observed in the acute ischemic phase underlie disturbances in voltage-gated channels6. A large body of evidence indicates that polyunsaturated fatty acids (PUFA) acylated in the phospholipids constituting the lipid bilayers of cell membranes modulate the activity of voltage-gated channels by either altering the biophysical membrane properties (indirect effect) or binding to the protein upon cleavage from cell membrane phospholipids (direct effect)7,8. The finding that dietary fats are readily incorporated into the cell membranes of cardiomyocytes put for the hypothesis that sustained dietary PUFA intake and the ensuing enrichment in cardiac phospholipids might reduce myocardial vulnerability to early PVF in MI9.
PUFA include mainly omega-3 (n-3) and omega-6 (n-6) fatty acids. n-3 PUFA, particularly those of marine origin (eicosapentaenoic acid [C20:5n-3, EPA] and docosahexaenoic acid [C22:6n-3, DHA]), have been shown to possess an array of cardioprotective effects10. Regarding n-6 PUFA, in particular linoleic acid (C18:2n-6, LA), although sustained intake of LA has been mechanistically linked to an increased low-density lipoprotein oxidation and to the transformation to arachidonic acid (C20:4n-6, AA—a precursor of proinflammatory lipid mediators), there is increasing evidence of the cardioprotective benefits of LA intake within the range advocated by the American Heart Association11. Experimental research uncovered a long time ago that replacement of saturated animal fat in the diet with either LA-rich or n-3-rich oils reduced the incidence and severity of arrhythmias occurring in ischemia9. However, this notion barely translated into clinical research. We hypothesized that in patients with ST-elevation myocardial infarction (STEMI), cardiac enrichment in specific fatty acids resulting from the consumption of fat-rich foods during the weeks prior to the event would influence the vulnerability of the myocardium to develop PVF. To address this issue, at hospital admission for STEMI, we performed lipidomics in 58 patients who developed PVF and in 116 non-PVF controls matched for cardiovascular risk factors, searching for associations between fatty acid species and incident PVF. Given that routine myocardial biopsy is not safe in the acute phase of STEMI, we determined fatty acidsin serum phospholipids, the status of which changes in parallel with heart phospholipids within a wide range of dietary fats12.
Results
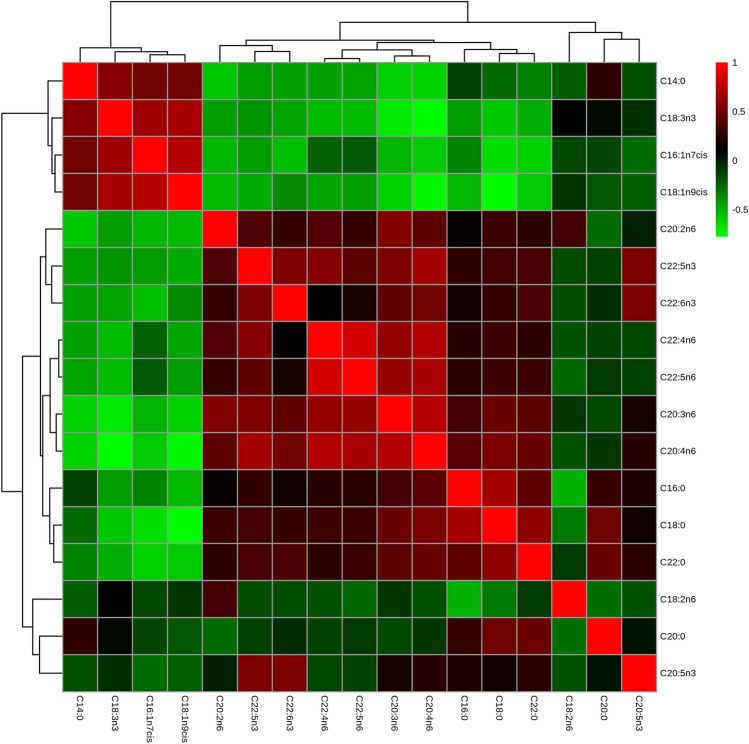
Table 1 provides the participants' characteristics by study group. Per the study design, we found no differences in age, sex, smoking status, or prevalence of treated dyslipidemia, diabetes mellitus or hypertension. We neither observed differences in plasma concentrations of total cholesterol, triglycerides or other known risk factors for PVF as advanced Killip-Kimball class or anterior infarct location. When comparing serum phospholipids status of main fatty acids at hospital admission by study group, significant differences were restricted to LA, with PVF patients showing a lower compared with matched controls (nominal P value = 0.002; false discovery rate [FDR]-corrected P value = 0.027; Table 2 and Fig. 1). When assessing the odds ratios (OR) for the prevalence of PVF associated with this PUFA, the unadjusted model (model 1) showed that each 1-standard deviation (SD) increase in the proportion of LA was related to a significant reduction (42%) in the prevalence of PVF (OR, 0.58; 95% confidence intervals [CI], 0.38 to 0.88; P = 0.01). The observed association remained essentially unchanged after the inclusion of confounders (Model 2, OR, 0.58; 95% CI, 0.37 to 0.90; P = 0.02). A non-linear pattern between LA and the odds of PVF was confirmed in an analysis using restricted cubic spline models that indicated a nadir of risk at 0 SD and decreasing odds of PVF at higher levels of LA (Fig. 2). Finally, the Spearman correlation coefficients for fatty acid species are shown in Fig. 3. We observed that n-6 PUFA were segregated in two groups. While ≥ 20-carbon species showed strong direct interrelationships (Spearman correlation coefficients ranging from 0.29 to 0.84), LA showed marginal and inverse associations with their longer-chain n-6 counterparts (Spearman correlation coefficient between LA and AA = − 0.17).
Table 1.
Baseline characteristics of primary ventricular fibrillation (PVF) cases and matched controls.
| Variable | PVF cases (n = 58) |
Non-PVF controls (n = 116) |
P-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 59.9 ± 13.1 | 59.9 ± 12.6 | 0.99 |
| Female | 7 (12.1) | 14 (12.1) | 1.00 |
| History | |||
| Smoking | 33 (56.9) | 65 (56.0) | 0.91 |
| Hypertension | 29 (50.0) | 58 (50.0) | 1.00 |
| Diabetes mellitus | 12 (20.7) | 25 (21.6) | 0.89 |
| Dyslipidemia | 35 (60.3) | 64 (55.2) | 0.52 |
| Cerebrovascular disease | 4 (6.9) | 9 (7.8) | 0.84 |
| Myocardial infarction | 8 (13.8) | 8 (6.9) | 0.14 |
| PCI | 8 (13.8) | 10 (8.6) | 0.29 |
| CABG | 0 (0) | 0 (0) | – |
| Physical examination | |||
| Killip-Kimball class III–IV | 6 (10.3) | 5 (4.3) | 0.12 |
| BMI, kg/m2 | 27.7 ± 5.2 | 27.3 ± 4.1 | 0.53 |
| Anterior infarct location | 29 (48.3) | 46 (38.7) | 0.22 |
| Angiography, ≥ 70% stenosis | 58 (100) | 114 (98.3) | 0.48 |
| 0 | 2 (3.4) | 0 (0) | 0.04 |
| 1 | 35 (60.3) | 60 (51.7) | 0.28 |
| 2 | 9 (15.5) | 28 (24.1) | 0.19 |
| 3 | 12 (20.7) | 26 (22.4) | 0.79 |
| Successful primary PCI | 55 (94.8) | 106 (91.4) | 0.42 |
| LVEF, % | 48.4 ± 10.6 | 51.8 ± 9.5 | 0.03 |
| Laboratory results | |||
| Hemoglobin, g/dL | 13.4 ± 1.6 | 13.0 ± 1.6 | 0.14 |
| eGFR, mL/min/1.73 m2 | 76.7 ± 30.6 | 86.4 ± 31.0 | 0.05 |
| Total cholesterol, mg/dL | 164.8 ± 36.0 | 175.9 ± 37.3 | 0.07 |
| Triglycerides, mg/dL | 114 (91–158) | 112 (57–144) | 0.49 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range: Q1–Q3). BMI, body mass index; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
P value obtained by χ2 test, Student’s t test or Wilcoxon rank sum test, as appropriate.
Table 2.
Proportion of fatty acids in serum phospholipids in primary ventricular fibrillation (PVF) cases and matched controls at hospital admission for STEMI.
| Fatty acid | PVF cases (n = 58) |
Non-PVF controls (n = 116) |
Nominal P-value | FDR-corrected P-value | Cohen’s d |
|---|---|---|---|---|---|
| C14:0 | 3.26 ± 2.09 | 2.90 ± 2.20 | 0.151 | 0.802 | − 0.17 |
| C16:0 | 29.06 ± 3.62 | 28.55 ± 4.00 | 0.379 | 0.802 | − 0.13 |
| C16:1n-7cis | 1.48 ± 1.21 | 1.33 ± 0.92 | 0.273 | 0.802 | − 0.15 |
| C18:0 | 13.44 ± 3.62 | 12.96 ± 3.06 | 0.487 | 0.802 | − 0.14 |
| C18:1n-9cis | 18.87 ± 6.65 | 18.16 ± 7.07 | 0.418 | 0.802 | − 0.10 |
| C18:2n-6 | 17.08 ± 3.27 | 19.20 ± 4.53 | 0.002 | 0.027 | 0.51 |
| C18:3n-3 | 0.28 ± 0.18 | 0.31 ± 0.42 | 0.513 | 0.802 | 0.06 |
| C20:0 | 0.14 ± 0.08 | 0.13 ± 0.07 | 0.605 | 0.802 | − 0.11 |
| C20:2n-6 | 0.29 ± 0.08 | 0.31 ± 0.09 | 0.250 | 0.802 | 0.19 |
| C20:3n-6 | 2.56 ± 0.99 | 2.67 ± 1.11 | 0.752 | 0.802 | 0.10 |
| C20:4n-6 | 8.62 ± 3.00 | 8.61 ± 3.35 | 0.668 | 0.802 | − 0.01 |
| C20:5n-3 | 0.60 ± 0.37 | 0.56 ± 0.37 | 0.366 | 0.802 | − 0.09 |
| C22:0 | 0.10 ± 0.05 | 0.12 ± 0.13 | 0.618 | 0.802 | 0.14 |
| C22:4n-6 | 0.31 ± 0.13 | 0.31 ± 0.12 | 0.804 | 0.802 | − 0.06 |
| C22:5n-3 | 0.22 ± 0.09 | 0.22 ± 0.10 | 0.610 | 0.802 | − 0.06 |
| C22:5n-6 | 0.55 ± 0.16 | 0.54 ± 0.17 | 0.587 | 0.802 | − 0.02 |
| C22:6n-3 | 3.14 ± 1.10 | 3.14 ± 1.23 | 0.755 | 0.802 | 0.00 |
Data are presented as mean ± standard deviation. Nominal P-values were assessed by Student’s t test, and the Benjamini–Hochberg procedure was applied on nominal P-values to control the false discovery rate (FDR).
Figure 1.
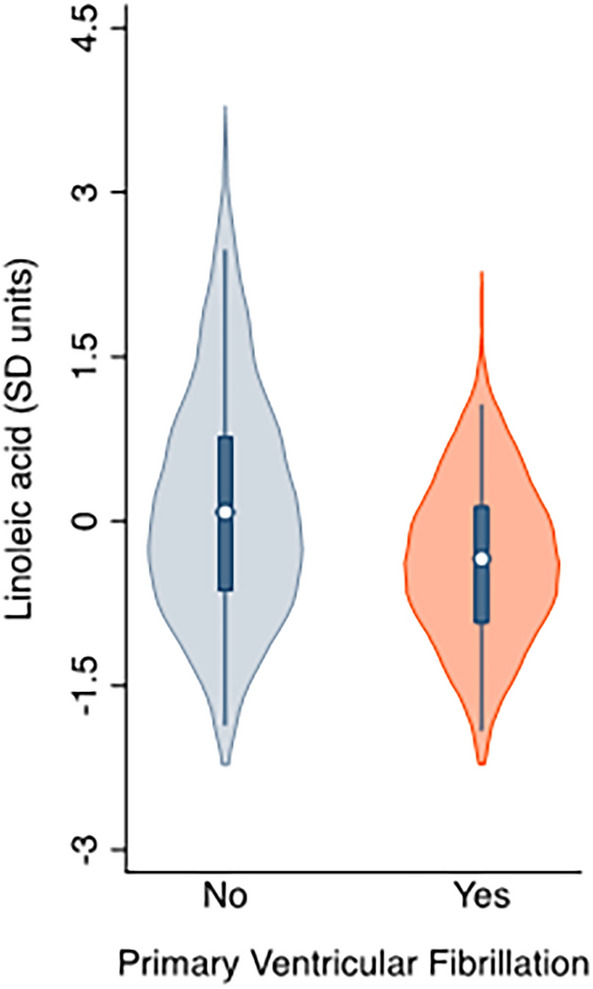
Serum phosphatidylcholine linoleic acid proportion by diagnosis of ventricular fibrillation.
Figure 2.
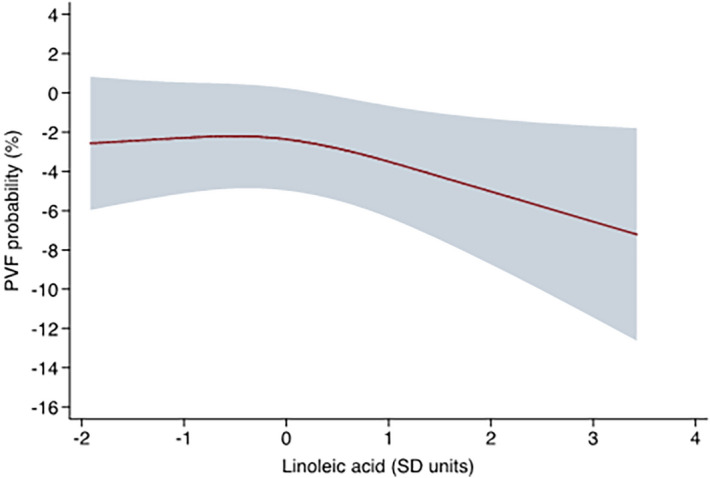
Adjusted predictions with the 95% confidence interval for the relationship between standardized linoleic acid (C18:2n-6) values and the occurrence of primary ventricular fibrillation (PVF). Standardized C18:2n-6 values were modeled by restricted cubic splines. All predictors were set to their mean values.
Figure 3.
Spearman correlation coefficients in all available fatty acid species in serum phospholipids.
Discussion
In this case–control, prospective study enrolling a large cohort of STEMI-driven PVF patients and matched non-PVF STEMI controls, we determined the fatty acid status of serum phospholipids at hospital admission for STEMI. These objective lipidomic biomarkers mirror not only dietary intake during the previous weeks, but also the fatty acid status in inner membranes, including cardiomyocytes. We found that a lower prevalence of PVF was associated with increasing levels of LA, an essential n-6 fatty acid naturally found in nuts (such as walnuts, pine nuts, pistachios and almonds) and seed oils (such as canola, corn, safflower, soybean, and sunflower oils).
Our findings are clinically relevant in two ways. First, we provide novel clinical evidence of a modifiable lifestyle (dietary) factor that is related to a lower risk of PVF, an endpoint that is a major contributor to short-term mortality in STEMI survivors. PVF is difficult to predict, and occurs at persistently high rates despite advances in the era of PPCI. Therefore, identifying any easily accessible and safe strategy to reduce the risk for PVF in STEMI patients is needed. Second, because LA in serum phospholipids reflects dietary levels of this essential fatty acid12,13, we suggest that intake of this n-6 PUFA may protect against PVF. This observation was repeatedly reported in animal models11 and may be mechanistically explained by changes in membrane cells14 upon incorporation of dietary LA into the phospholipids, as occurs with other dietary PUFA12. Notably, because LA can be transformed into AA, a substrate of many proinflammatory lipid mediators, dietary LA is widely perceived to promote inflammation, contributing to cardiovascular disease. Although LA can be converted to a few inflammatory and vasoconstrictory lipid mediators15, there is a growing body of evidence for the potential cardio-metabolic benefits of LA16, including a recent landmark study pooling data from 30 prospective cohorts, which reported that higher in vivo circulating and tissue levels of LA were associated with lower risk of major cardiovascular events17. Our data contribute to countering the demand to remove LA from the diet based on its proposed harmful effects. Indeed, seed oils that used to be rich sources of LA, such as safflower and sunflower oils, have now been hybridized to substantially remove LA. According to a recent modeling experiment, this shift may be placing the population (children in particular) at increased risk of a deficiency in essential fatty acids, including LA18. Studies such as ours may help build a firmer evidence base for the benefits of LA, which will hopefully slow (or even reverse) these well-intentioned but, in our view, misguided efforts to remove LA from the diet.
Interestingly, we failed to find significant associations for marine-derived n-3 fatty acids. Though a large body of observational evidence exists on the benefits of dietary EPA and DHA against sudden cardiac death, the issue of whether dietary EPA and DHA may protect against PVF in the setting of STEMI remains unsettled19, with randomized controlled trials conducted in patients with chronic myocardial scars wearing implantable cardioverter defibrillators who already have a history of ventricular arrhythmia20–22. Further research is warranted to clarify effects of dietary n-3 PUFA on PVF.
This study had several limitations. First, its observational nature precludes establishing causality between circulating LA (or dietary LA intake) and prevention of PVF in the setting of STEMI. Such a link could only be confirmed by a randomized controlled trial of dietary supplementation with LA before the occurrence of STEMI—which practically speaking would be challenging to design. Second, we used the fatty acid profile of serum phospholipids. Although the use of this lipidomic-based objective biomarker of long-term dietary fatty acid intake circumvents the disadvantages of self-reported dietary data (i.e., food diaries and food frequency questionnaires), the fatty acid profile of serum phospholipids does not reflect long-term intake as accurately as the fatty acids in adipose tissue or red blood cells. Third, dietary LA in Mediterranean populations is largely supplied by nuts and seeds23, which contain many cardioprotective bioactive agents in addition to PUFA24. Therefore, we cannot rule out the parent foods themselves being the actual protective agent and LA just a marker of their consumption. Finally, because some potential health-related confounding variables (i.e., socioeconomic status, education, adherence to Mediterranean diet) were not available, we could not exclude the possibility that uncaptured environmental factors may have influenced or caused the observed association.
In conclusion, we identified an association of elevated LA in serum phospholipids at the time of STEMI with a lower risk of PVF. Thus, sustained consumption of sources rich in LA may reduce the risk of ischemia-driven cardiac arrest. Our results, which concur with experimental data and suggested membrane-based benefits ascribed to dietary LA, contribute to dispel the notion that the entire n-6 family of fatty acids promote cardiovascular disease.
Methods
Study design and population
The study was a post-hoc, nested case–control study of participants included in the Ruti-STEMI biomarkers study between February 23, 2011, and January 30, 2016. The study included prospectively consecutive patients with STEMI in a stable and well-defined geographic area of approximately 850,000 inhabitants in the northern metro area of Barcelona in Catalonia, Spain, within the Codi IAM primary percutaneous coronary intervention (PPCI) network. For the current analysis, only patients having a blood sample available for fatty acid measurement25 were considered (n = 944).
Cases (n = 58) consisted of all individuals diagnosed with PVF during the acute phase of STEMI. Controls were STEMI patients who did not develop PVF during the first 48 h after symptom onset. They were selected by matching two controls with each case using the following criteria: age (± 3 years), sex, smoking status, dyslipidemia, diabetes mellitus, and hypertension. A total of 116 controls were matched to cases (Supplementary Fig. S1).
The STEMI diagnosis was established according to the current universal definition of MI at the time of the study3, which included chest pain and electrocardiogram showing ST-segment elevation in two or more contiguous leads (minimum 0.1 mV in the frontal leads and 0.2 mV in the precordial leads) or with new-onset left bundle branch block3. Baseline demographics and clinical data were recorded during hospital admission.
In our present analysis, patients were classified according to whether they developed PVF in the first 48 h and included in-hospital (if occurring within hospital facilities before, during, or after PPCI up to 48 h after MI diagnosis) and out-of-hospital PVF (within the EMS system) when arriving alive to the hospital. Events beyond 48 h likely include secondary ventricular fibrillation in a dysfunctional myocardium and were not considered here.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the local Ethics Committee (The Ethics Committe of the Clinical Investigation of Germans Trias i Pujol Hospital, reference EO-11-061). Patients or their representatives provided written informed consent.
Laboratory measurements
Blood samples were obtained from veno-puncture soon after admission and within 12 h after symptom onset. Samples were processed in a central laboratory to measure biomarkers. Serum was stored at − 80 °C until fatty acid analysis. Total serum lipids were extracted with 2 mL of chloroform/methanol (2:1 v/v). After shaking, the mixture was centrifuged (5 min at 3500 rpm at room temperature), and the organic phase was transferred to a borosilicate glass tube and evaporated to dryness under N2 at 30ºC. Phosphatidylcholine was isolated by solid-phase extraction as described in Burdge et al.26. Briefly, dried serum lipid extract was redissolved in dry chloroform and applied to an aminopropyl silica column (Sep-Pak Vac NH2 cartridges, 100 mg packed silica per 1 mL, Waters) under gravity. After eluting neutral lipid fractions (namely cholesteryl esters and triglycerides), we collected phosphatidylcholine upon elution with a mixture of dry chloroform and methanol. The fraction was evaporated to dryness under N2 at 30 °C, and fatty acids were then hydrolyzed and methylated by acidified methanol26. Briefly, 1 mL of dry methanol containing H2SO4 (2%) was added to the tube, which was capped and placed into a block heather (50 °C) for 2 h. After cooling, 80 µL of a solution containing K2CO3 and 500 µL of n-hexane were added. The tubes were shaken for 1 min, and then centrifuged for 5 min at 3500 rpm at room temperature to separate the layers. The hexane layer was transferred into an automatic injector vial equipped with a glass insert of 300 µL. Fatty acid methyl esters were injected to an Agilent HP 7890 Gas Chromatograph equipped with a 30 m × 0.25 µm × 0.25 mm SupraWAX-280 capillary column (Teknokroma, Barcelona, Spain), an autosampler, and flame ionization detection. Gas-chromatography was run using an optimized temperature program, as follows: the program started at 50 °C, held for 2 min and increased to 220 °C at a rate of 4 °C/min. Helium was used as a carrier gas (30 psi, constant pressure mode). Temperature of injector and detector was 260 °C. The amount of each fatty acid is expressed as a percentage of the total amount of fatty acids.
Statistical analysis
Summary statistics including counts and percentages are provided for categorical variables and both means with standard deviations and medians with interquartile ranges are provided for continuous variables. Categorical variables were compared using the χ2 test. Continuous variables were compared using the Student’s t test or Wilcoxon rank sum test. Differences between PVF cases and matched controls for log-transformed fatty acids of serum phospholipids (C14:0, C16:0, C16:1n-7cis, C18:0, C18:1n-9cis, C18:2n-6, C18:3n-3, C20:0, C20:2n-6, C20:3n-6, C20:4n-6, C20:5n-3, C22:0, C22:4n-6, C22:5n-3, C22:5n-6; C22:6n-3) were assessed by Student’s t test with effect sizes calculated by Cohen’s d-test, and the Benjamini–Hochberg procedure was applied on nominal P-values to control the FDR27. An FDR-corrected P value of < 0.05 was considered statistically significant. The ORs with confidence intervals CIs for PVF associated to PUFA surviving the adjustment by the FDR procedure were assessed by conditional logistic regression model. In addition to an unadjusted model (model 1), a model adjusted for left ventricular ejection fraction, Killip-Kimball class and anterior infarct location (y/n) was also constructed (Model 2). ORs reflecting a change of one SD in a given fatty acid and the CIs are reported. Finally, the interrelationship between selected serum phospholipids PUFA was assessed using the Spearman rank correlation and plotted in heatmap format.
Differences were considered significant if P < 0.05. All analyses were performed in STATA V.13.0 (StataCorp, College Station, TX) or R Software version 4.0.328.
Supplementary Information
Acknowledgements
We wish to acknowledge the physicians and nurses of the Coronary Care Unit of the Hospital Germans Trias i Pujol for their continuous dedicated care of the patients with STEMI and their careful collection of clinical and biological data. CIBEROBN and CIBERCV are initiatives of Instituto de Salud Carlos III, Spain.
Author contributions
I.L., A.S.-V. and A.B.-G. designed research; T.O., I.L., F.R., G.C., F.M.-G., O.J.P., C.G.-G., A.S.-V. and A.B.-G. conducted research; G.C. and F.M.-G. analyzed data or performed statistical analysis; T.O., I.L., D.L.B., M.F., W.S.H., A.S.-V. and A.B.-G. wrote the paper; A.B.-G. had primary responsibility for the final content. All the authors have read and approved the final manuscript.
Funding
Part of this work was supported by a grant from the California Walnut Commission (Folsom, CA, USA; to AS-V). AB-G is supported by ISCIII (PI17/01487, PI18/00256, and PIC18/00014) and CIBER Cardiovascular (CB16/11/00403) projects as a part of the National R&D&I Plan, and co-funded by ISCIII-Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (ERDF). The funding agencies had no input in the study design, data collection, analyses, or writing and submission of the manuscript.
Data availability
Data described in the article will be made available upon request pending application and approval.
Competing interests
DLB discloses the following relationships-Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. WSH holds an interest in OmegaQuant Analytics, a lab that offers blood fatty acid testing; and is a member of the RB Schiff Science and Innovation Advisory Board. AS-V reports grants and support from the California Walnut Commission to attend professional meetings. AB-G reports grants and personal fees from AstraZeneca, Abbott, Boehringer-Ingelheim, Vifor, Novartis, Roche Diagnostics, and Critical Diagnostics. TO, IL, FR, GC, MF, FM-G, OJP and CG-G have no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Teresa Oliveras and Iolanda Lázaro.
Contributor Information
Teresa Oliveras, Email: 3aoliveras@gmail.com.
Aleix Sala-Vila, Email: asv@faresinst.com.
Antoni Bayés-Genís, Email: abayesgenis@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08453-0.
References
- 1.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CA, et al. Changes over time in the incidence and case-fatality rates of primary ventricular fibrillation complicating acute myocardial infarction: Perspectives from the Worcester Heart Attack Study. Am. Heart. J. 2000;139:1014–1021. doi: 10.1067/mhj.2000.106160. [DOI] [PubMed] [Google Scholar]
- 3.García-García C, et al. Trends in short- and long-term ST-segment-elevation myocardial infarction prognosis over 3 decades: A Mediterranean population-based ST-segment-elevation myocardial infarction registry. J. Am. Heart. Assoc. 2020;9:e017159. doi: 10.1161/JAHA.120.017159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CX, et al. Epidemiology of sudden cardiac death: Global and regional perspectives. Heart. Lung. Circ. 2019;28:6–14. doi: 10.1016/j.hlc.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Bougouin W, et al. Incidence of sudden cardiac death after ventricular fibrillation complicating acute myocardial infarction: A 5-year cause-of-death analysis of the FAST-MI 2005 registry. Eur. Heart. J. 2014;35:116–122. doi: 10.1093/eurheartj/eht453. [DOI] [PubMed] [Google Scholar]
- 6.Sattler SM, et al. Ventricular arrhythmias in first acute myocardial infarction: Epidemiology, mechanisms, and interventions in large animal models. Front. Cardiovasc. Med. 2019;6:158. doi: 10.3389/fcvm.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohannon BM, et al. Polyunsaturated fatty acid analogues differentially affect cardiac NaV, CaV, and KV channels through unique mechanisms. Elife. 2020;9:e51453. doi: 10.7554/eLife.51453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson JE, Frampton DJA, Liin SI. Polyunsaturated fatty acids as modulators of KV7 channels. Front. Physiol. 2020;11:641. doi: 10.3389/fphys.2020.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charnock JS. Lipids and cardiac arrhythmia. Prog. Lipid. Res. 1994;33:355–385. doi: 10.1016/0163-7827(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Budoff MJ, Mason RP. A revolution in omega-3 fatty acid research. J. Am. Coll. Cardiol. 2020;76:2098–2101. doi: 10.1016/j.jacc.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 12.Abbott SK, Else PL, Atkins TA, Hulbert AJ. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta. 2012;1818:1309–1317. doi: 10.1016/j.bbamem.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid. Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, et al. Common lipid features of lethal ventricular tarchyarrhythmias (LVTAs) induced by myocardial infarction and myocardial ion channel diseases. Sci. Rep. 2017;7:4220. doi: 10.1038/s41598-017-04620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marangoni F, et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:90–98. doi: 10.1016/j.atherosclerosis.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Marklund M, et al. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139:2422–2436. doi: 10.1161/CIRCULATIONAHA.118.038908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raatz SK, Conrad Z, Jahns L, Belury MA, Picklo MJ. Modeled replacement of traditional soybean and canola oil with high-oleic varieties increases monounsaturated fatty acid and reduces both saturated fatty acid and polyunsaturated fatty acid intake in the US adult population. Am. J. Clin. Nutr. 2018;108:594–602. doi: 10.1093/ajcn/nqy127. [DOI] [PubMed] [Google Scholar]
- 19.Albert CM. Omega-3 fatty acids, ventricular arrhythmias, and sudden cardiac death: Antiarrhythmic, proarrhythmic, or neither. Circ. Arrhythm. Electrophysiol. 2012;5:456–459. doi: 10.1161/CIRCEP.112.971416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raitt MH, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: A randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 21.Leaf A, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer IA, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur. Heart. J. 2009;30:820–826. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadatian-Elahi M, et al. Plasma phospholipid fatty acid profiles and their association with food intakes: Results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2009;89:331–346. doi: 10.3945/ajcn.2008.26834. [DOI] [PubMed] [Google Scholar]
- 24.Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: Epidemiological and clinical trial evidence. Circulation. 2013;128:553–565. doi: 10.1161/CIRCULATIONAHA.112.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lázaro I, et al. Circulating omega-3 fatty acids and incident adverse events in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2020;76:2089–2097. doi: 10.1016/j.jacc.2020.08.073. [DOI] [PubMed] [Google Scholar]
- 26.Burdge GC, Wright P, Jones AE, Wootton SA. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000;84:781–787. doi: 10.1017/S0007114500002154. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. R. Found. Stat. Comput. (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article will be made available upon request pending application and approval.