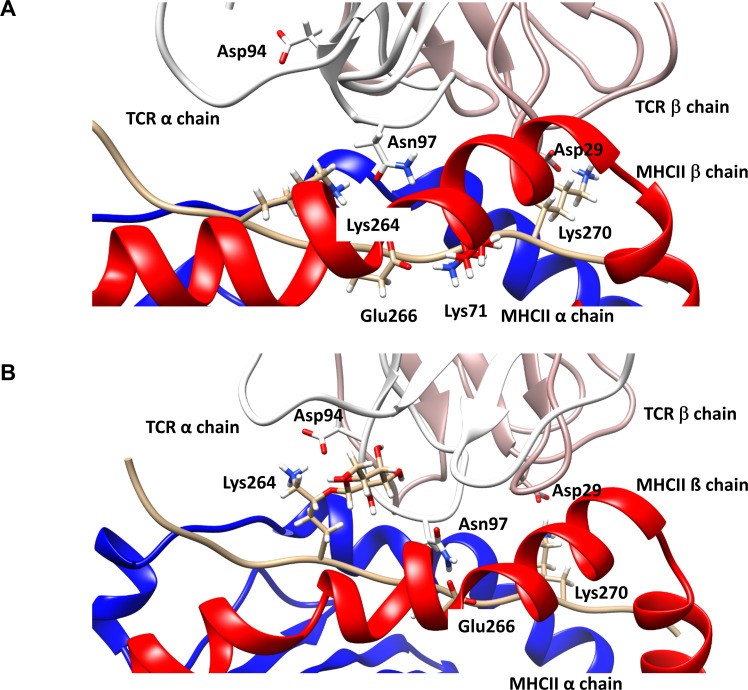
Figure 6.
Geometry of the trimolecular complexes after 950 ns of molecular dynamics simulation. (A) Trimolecular complex consisting of TCR#16 and DRB1*04:01 complexed with the unmodified Col2259-273 peptide. The initial salt bridge Lys264 - Asp94 (TCRα-CDR3) breaks to allow for neoformation of a bond between Lys264 of Col2259-273 and Asn97 (TCRα-CDR3). Glu266 of Col2259-273, which initially interacted with Asn97 in the TCRα (figure 5), now forms a salt bridge with Lys71 in the β-chain of DRB1*04:01, whereas the initial bond between Lys270 (Col2259-273)-Asp29 (TCRβ-CDR1) remains preserved. (B) Trimolecular complex consisting of TCR#16 and DRB1*04:01 complexed with the gal264Col2259-273 peptide. The initial salt bridge Lys264 - Asp94 (TCRα-CDR3) remains intact, likely due to the stabilising impact of the sugar ring by decreasing the mobility of Lys264. The galactose ring in close contact to the CDR3 backbone of TCRα forms a hydrogen bond to the backbone. The two other side chain bonds of the starting geometry (figure 5), Glu266 (gal264Col2259-273)-Asn97 (TCRα-CDR3) and Lys270 (gal264Col2259-273)-Asp29 (TCRβ-CDR1), remain detectable. CDR, complementarity determining region; MHC, major histocompatibility complex; TCR, T-cell receptor.