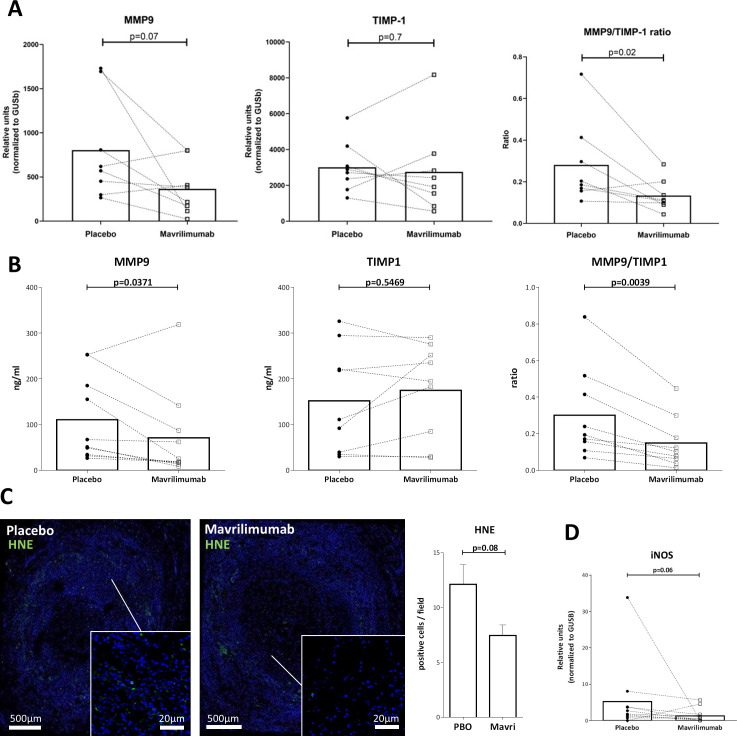
Figure 7.
Effect of mavrilimumab on molecules related to vascular injury. (A) Transcripts of MMP-9, tissue inhibitor of metalloproteinases 1 (TIMP-1) and MMP-9/TIMP-1 mRNA ratio in 8 GCA-positive temporal arteries cultured with placebo or mavrilimumab. (B) MMP-9, TIMP-1 protein concentration and MMP-9/TIMP-1 protein ratio in the corresponding supernatants (ng/mL). (C) Immunofluorescence staining of HNE (green) with nuclei (in blue) in a GCA-involved artery cultured with placebo or mavrilimumab, and its quantitation (right panel). Immunofluorescence was performed in two GCA cultured arteries, with consistent results. (D) NOS2 transcripts in 11 cultured GCA arteries exposed to placebo or mavrilimumab. GCA, giant cell arteritis; HNE, 4-hydroxynonenal; MMP-9, matrix metalloprotease 9.