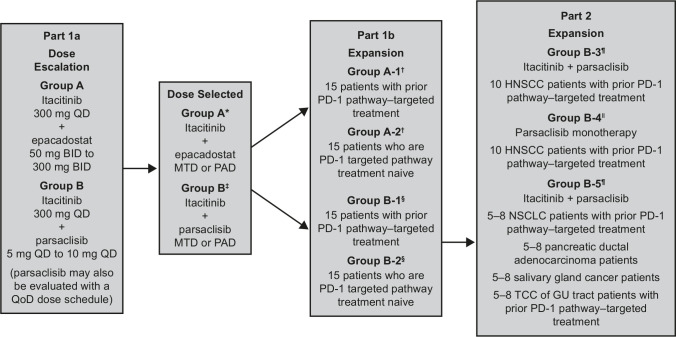
Figure 1.
Study design. *Group A included three dose levels: itacitinib 300 mg once a day plus epacadostat 50 mg two times per day, itacitinib 300 mg once a day plus epacadostat 100 mg two times per day, and itacitinib 300 mg once a day plus epacadostat 300 mg two times per day. †Treatment for groups A-1 and A-2 was itacitinib 300 mg once a day plus epacadostat 300 mg two times per day. ‡Group B included seven dose levels: itacitinib 300 mg once a day plus parsaclisib 2.5 mg once every other day, itacitinib 300 mg once a day plus parsaclisib 5 mg once a day, itacitinib 300 mg once a day plus parsaclisib 10 mg once a day, itacitinib 100 mg once a day plus parsaclisib 0.3 mg once a day, itacitinib 100 mg once a day plus parsaclisib 1 mg once a day, itacitinib 300 mg once a day plus parsaclisib 0.3 mg once a day, and itacitinib 300 mg once a day plus parsaclisib 1 mg once a day. §Treatment for groups B-1 and B-2 was itacitinib 300 mg once a day plus parsaclisib 10 mg once a day. ¶Treatment for groups B-3 and B-5 was itacitinib 100 mg once a day plus parsaclisib 0.3 mg once a day. ǁTreatment for group B-4 was parsaclisib 0.3 mg once a day monotherapy; one patient in group B-4 had itacitinib 100 mg once a day added, per protocol, due to disease progression. All patients receiving parsaclisib plus itacitinib (except parsaclisib 0.3 mg once a day plus itacitinib 100 mg once a day) were required to receive a standard Pneumocystis jirovecii prophylaxis regimen determined by the investigator. BID, two times per day; GU, genitourinary; HNSCC, head and neck squamous cell carcinoma; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; PAD, pharmacologically active dose; PD-1, programmed cell death-1; QD, once a day; QoD, once every other day; TCC, transitional cell carcinoma.