Figure 4.
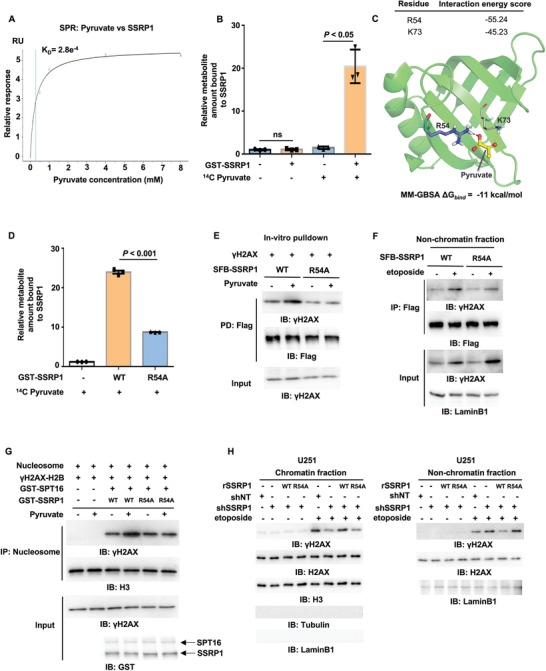
The binding of pyruvate to SSRP1 is required for FACT‐mediated γH2AX loading to chromatin. IP and IB analyses were performed with indicated antibodies. Data are representative of at least three independent experiments. A) SPR (surface plasmon resonance) assay was performed with bacteria‐purified recombinant SSRP1 protein (100 µg mL–1) and increasing doses of pyruvate. K D: dissociation constant (pyruvate versus SSRP1). B) Metabolite–protein binding assay. Bacteria‐purified recombinant GST‐SSRP1 was incubated with 14C‐labeled pyruvate. SSRP1‐bound pyruvate was quantified by scintillation counting. Data represent the mean ± SD of the relative metabolite amount bound to SSRP1 from three independent experiments (two‐tailed Student's t‐test). C) A representative image of the structure of human SSRP1 bound to pyruvate was shown. Atoms of pyruvate are presented as balls and sticks with carbon atoms in yellow and oxygen atoms in red. The whole protein is shown as a cartoon in green, while R54 is shown as sticks with purple carbon atoms and K73 is shown as lines with gray carbon atoms. Red dashed lines represent salt‐bridge and yellow ones for hydrogen bonds. The binding energy (ΔG bind) of pyruvate with SSRP1 calculated using MM‐GBSA is ‐11 kcal mol–1. D) Metabolite–protein binding assay. Bacteria‐purified recombinant GST‐SSRP1 WT or R54A was incubated with 14C‐labeled pyruvate. The pyruvate bound to SSRP1 WT or R54A was quantified by scintillation counting. Data represent the mean ± SD of the relative metabolite amount bound to SSRP1 from three independent experiments (two‐tailed Student's t‐test). E) SSRP1 WT or R54A was immunoprecipitated and purified from U251 cells stably expressing SFB‐SSRP1 WT or R54A. γH2AX was immunoprecipitated and purified from etoposide‐treated U251 cells. The in vitro pulldown experiment was performed by incubating the purified SSRP1 WT or R54A with purified γH2AX in the absence or presence of pyruvate (1 × 10–3 m). F) HEK293T cells were transfected with SFB‐SSRP1 WT or R54A and then treated with or without etoposide (200 × 10–6 m, 1 h). Nonchromatin‐bound fraction was prepared. G) In vitro histone exchange assay. H2AX‐H2B dimers were phosphorylated by purified recombinant monomeric ATM to obtain γH2AX‐H2B dimers. Commercially purchased nucleosomes (120 ng) were incubated with γH2AX‐H2B dimers, GST‐SPT16 and GST‐SSRP1 WT or R54A in the absence or presence of pyruvate (1 × 10–3 m) for 1 h. The recruitment of γH2AX to the chromatin was determined by IB analysis of immobilized nucleosomes with anti‐γH2AX antibody. Histone H3 was used as loading control. H) U251 cells were infected with the lentivirus expressing shNT or shSSRP1 and the SSRP1‐depleted U251 cells were reconstituted with or without the expression of rSSRP1 WT or R54A. These cells were then treated with or without etoposide (200 × 10–6 m, 1 h). Chromatin fraction (left panel) and non‐chromatin‐bound fraction (right panel) were prepared.
