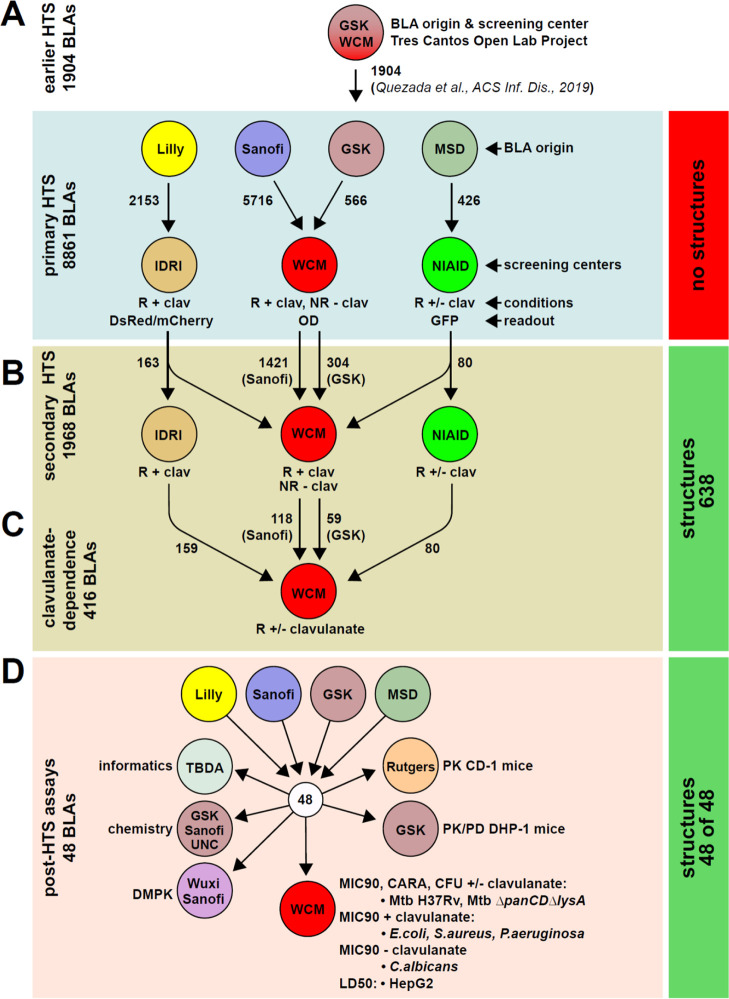
Figure 1.
Schematic of the β-lactam screening strategy. (a) β-lactams were sent from four pharma partners to three academic screening centers to test for activity against wild-type H37Rv or ΔpanCDΔlysA Mtb that was replicating or rendered nonreplicating in a four-stress model.48,50 Primary screening hit rates are shown in Table S1. (b) Subset of actives from Sanofi and GSK were resupplied as fresh stocks to a single academic screening center (WCM) for retesting head-to-head against Mtb ΔpanCDΔlysA at WCM. The GSK primary and secondary screens included some β-lactams from an earlier screen of their β-lactam collection.47 Actives from Lilly and MSD were retested at the IDRI and NIAID, respectively. (c) 416 confirmed actives from all four companies were tested at WCM head-to-head for clavulanate dependency in the dose–response format against replicating Mtb ΔpanCDΔlysA. (d) Final set of 48 β-lactams supplied for PK, DMPK, chemistry, and phenotypic testing. The annotation on the right of the figure describes the structure reveal: in (a), no structures were disclosed; in (b,c), 638 structures were revealed to all parties; and in (d), the final 48 compounds were all assigned a structure (primarily those that were not assigned in (b,c). Abbreviations: GSK, Diseases of the Developing World at GlaxoSmithKline; IDRI, Infectious Disease Research Institute; WCM, Weill Cornell Medicine; NIAID, National Institute of Allergy and Infectious Diseases; UNC, University of North Carolina; TBDA, Bill & Melinda Gates TB Drug Accelerator; R, replicating conditions; NR, 4-stress nonreplicating conditions; clav, clavulanate at 4 μg/mL; DsRed/mCherry, red fluorescent protein; GFP, green fluorescent protein; and OD, optical density at A580.