Abstract
Purpose
Half of the surgically proven Rathke’s cleft cysts (RCCs) can be preoperatively misdiagnosed as cystic pituitary adenoma (CPA). We aimed to evaluate the usefulness of contrast-enhanced (CE) 3D T2 fluid-attenuated inversion-recovery (3D T2-FLAIR) imaging for differentiating between CPA and RCC.
Methods
This retrospective study included six patients with RCC (all pathologically confirmed) and six patients with CPA (five pathologically confirmed, one clinically diagnosed). The 12 patients underwent pre- and post-contrast T1-weighted (T1W)- and 3D T2-FLAIR imaging at 3T. Based on the degree of enhancement of the lesion wall, two radiologists independently scored the images using a 3-point grading system. Interobserver agreement was calculated by using the κ coefficient. The statistical significance of grading differences was analyzed with the Mann–Whitney U-test. Another neuroradiologist first interpreted conventional MR images (1st session), and then the reader read images to which the 3D T2-FLAIR images had been added (2nd session). Sensitivity, specificity, and accuracy of the reader’s interpretation were calculated.
Results
Interobserver agreement for post-contrast T1W- and 3D T2-FLAIR images was excellent (κ = 1.000 and 0.885, respectively). Although the mean enhancement grade on post-contrast T1W images of RCCs and CPAs was not significantly different, on post-contrast 3D T2-FLAIR images it was significantly higher for RCCs and CPAs (P < 0.05). Three CPAs (50%) showed remarkable, donut-like enhancement along the inner margin of the cyst on CE-3D T2-FLAIR images; this was not the case on CE-T1W images. The sensitivity, specificity, and accuracy of the 2nd session were 1.00, 0.83, and 0.92, respectively, which were improved compared to the 1st session (1.00, 0.50, and 0.75, respectively).
Conclusion
CE-3D FLAIR imaging is useful for discriminating CPAs and RCCs.
Keywords: Rathke’ cleft cyst, cystic pituitary adenoma, 3D T2 fluid-attenuated inversion-recovery imaging
Introduction
Pituitary adenomas are benign tumors of the adenohypophysis; cystic changes are common. Two hypotheses have been proposed for this change: 1) ischemia and necrosis of the adenoma tissue and 2) changes secondary to intratumoral hemorrhage.1–4 Pituitary adenomas with cystic and/or hemorrhagic changes show different intensity on T1- and T2-weighted images.5–9
Rathke’s cleft cyst (RCC) is a benign epithelial cyst thought to originate from the remnants of the Rathke pouch; it arises in the region of the pars intermedium and can extend to suprasellar location.10 RCCs account for 6–10% of symptomatic sellar and suprasellar lesions.11 Similar to cystic pituitary adenomas (CPAs), depending on their cystic content, RCCs show different signal intensity on T1- and T2-weighted images.12
The differentiation between CPAs and RCCs is important for treatment planning.13,14 Symptomatic CPAs require total resection or medication, whereas surgical drainage is the mainstay for symptomatic RCCs.15,16 Characteristic MRI features such as a fluid-fluid level, septation, an off-midline location, and the presence of an intracystic nodule, can help to discriminate CPAs from RCCs.1,10,17,18 On the other hand, Wen et al.19 reported that 50% of surgically proven RCCs were preoperatively misdiagnosed as CPA. According to Hua et al.,20 the signal intensity of the cystic fluid does not help in distinguishing non-neoplastic cysts from cystic neoplasms, but cystic wall enhancement may be useful to discriminate neoplastic from non-neoplastic lesions. However, many RCCs are surrounded by the enhancing normal pituitary gland and mimic the wall enhancement of cystic lesions.21 This renders the differentiation of CPAs from RCCs difficult.21
The contrast-enhanced (CE) 3D T2 fluid-attenuated inversion-recovery (3D T2-FLAIR) MR imaging sequence is sensitive to low concentrations of gadolinium and flow compared with T1-weighted images.22 In the previous experimental study, 3D T2-FLAIR sequence exhibited higher sensitivity to low Gd concentrations and lower sensitivity to high Gd concentrations than did 2D T1-weighted (T1W) images and magnetization prepared rapid acquisition with gradient echo (MPRAGE) sequences.22 Additionally, for 3D T2-FLAIR, the signal was almost not seen in the flow velocity of more than 1.0 cm/second and the signal intensity of Gd in CE 3D T2-FLAIR decreased as increasing the flow velocity.22 Azuma et al.23 recently reported that differences in the wall enhancement of RCCs and cystic craniopharyngiomas on CE 3D T2-FLAIR images were helpful for their differentiation. Similar to this study, we hypothesized that CE 3D T2-FLAIR imaging is useful for differentiating between CPAs and RCCs. The purpose of our study was to verify the hypothesis using the MR imaging data available at our institution.
Materials and Methods
Study population
The institutional review board of the University of Miyazaki Hospital approved our retrospective, observational study (No. 2020-176), and the requirement for informed consent was waived.
We included MR imaging data of 47 consecutive patients with RCC or pituitary adenoma who underwent MR examination including pre- and post-contrast 3D FLAIR between October 2018 and August 2020 using a 3T MRI scanner (Ingenia; Philips Healthcare, Best, the Netherlands). The excluded were 10 patients with RCC without pathologically confirmation and 25 patients with pituitary adenoma with apparent solid component. Finally, our study population consisted of 12 patients (6 men, 6 women; age range 39–83 years; mean age 60 years): 6 harbored pathologically confirmed RCCs, 5 pathologically confirmed CPA, and 1 clinically diagnosed CPA. In our study, CPA was defined as a pituitary adenoma with no apparent solid component on MR images. In one case with clinically diagnosed pituitary adenoma, her serum prolactin level was 114.5 ng/ml, and the other pituitary hormones were in the normal range. Drug-induced prolactinoma was denied. After dopamine agonist medication, the serum prolactin level fell to within the normal range (17.5 ng/ml). All 12 patients had undergone conventional pre- and post-contrast 2D T1W- and 3D T2-FLAIR imaging studies on a 3T unit.
MR protocol
All images were obtained on the 3T MRI scanner (Philips Healthcare) using a 32-channel head coil. The following pulse sequences were acquired before contrast agent administration: sagittal and coronal 2D T1W imaging (TR, 450 ms; TE, 10 ms; section thickness, 2.5 mm; matrix, 256 × 192; FOV, 170 × 170 mm; imaging time, 3 min 28 sec), sagittal and coronal 2D T2-weighted (T2W) imaging (TR, 3000 ms; TE, 80 ms; section thickness, 2.5 mm; matrix, 352 × 262; FOV, 170 × 170 mm, imaging time, 2 min) and sagittal 3D T2-FLAIR imaging (TR, 4500 ms; TE, 252 ms; IR, 1600 ms; section thickness, 1.25 mm; matrix, 224 × 168; FOV, 170 × 170 mm; imaging time: 1 min 57 sec). To reduce the scan time, we applied compressed sensing (CS) for 3D T2-FLAIR sequences; CS-sensitivity encoding (CS-SENSE) was 4.0. Coronal multiplanar reconstructed images were generated for 3D T2-FLAIR sequences. Sagittal 2D T1W- and 3D T2-FLAIR imaging were performed after contrast agent administration. The contrast agent (Gadobutrol; Bayer HealthCare Pharmaceuticals, Berlin, Germany) was intravenously administered at 0.1 mmol/kg body weight. Post-contrast MR studies were started about 60–120 sec after contrast material injection and obtained in the order of sagittal and coronal 2D T1W- and 3D T2-FLAIR imaging.
Image evaluation
Two radiologists (readers 1 and 2, with 6 and 10 years of the experiences as radiologists, respectively) were blinded to the patient identity and the final diagnosis. They independently graded the degree of contrast enhancement of the cyst wall on T1W- and 3D T2-FLAIR images on a PACS (ShadeQuest/ViewR-DG; FUJIFILM, Tokyo, Japan) workstation. They used a 3-point scoring system where grade 2 = most of the cyst wall and/or cyst inner margin enhanced, grade 1 = some of the cyst wall and/or cyst inner margin enhanced, and grade 0 = no enhancement in the cyst wall and cyst inner margin). Final judgments were obtained by consensus of the two readers.
One experienced neuroradiologist with 30 years of reading experience conducted a blinded reading study. Each case was subjected to two reading sessions using a PACS workstation. This reader evaluated the conventional MR images to diagnose the two types of pituitary lesions at the 1st session where sagittal views of pre- and post-contrast T1W images, and T2W images were presented. The reader assessed the lesions using a 5-point confidence scale, where 1 = definitely RCC, 2 = probably RCC, 3 = equivocal, 4 = probably CPA, and 5 = definitely CPA. After the 1st session, the reader performed a 2nd reading session where sagittal views of pre- and post-contrast 3D T2-FLAIR images were added. The reader then reassessed the lesions using the same 5-point confidence scale. After the blinded study, the reader retrospectively identified the reason(s) for the incorrect image interpretation.
Statistical analysis
All statistical analyses were performed with MedCalc version 19.2.1 (MedCalc Software, Mariakerke, Belgium). Interobserver agreement was assessed with the Cohen kappa (κ) coefficient where 0.81–1.00 = excellent-, 0.61–0.80 = good-, 0.41–0.60 = moderate-, 0.21–0.40 = fair-, and 0.00–0.20 = poor agreement. The statistical significance of grading differences was analyzed with the Mann–Whitney U-test. Differences of P < 0.05 were considered statistically significant.
Sensitivity, specificity, and accuracy of the reader’s 1st and 2nd interpretation were calculated. For this evaluation, we defined that the lesions with a confidence score of 1 or 2 were considered as RCC; those with a score of 3, 4, or 5 as CPA.
Results
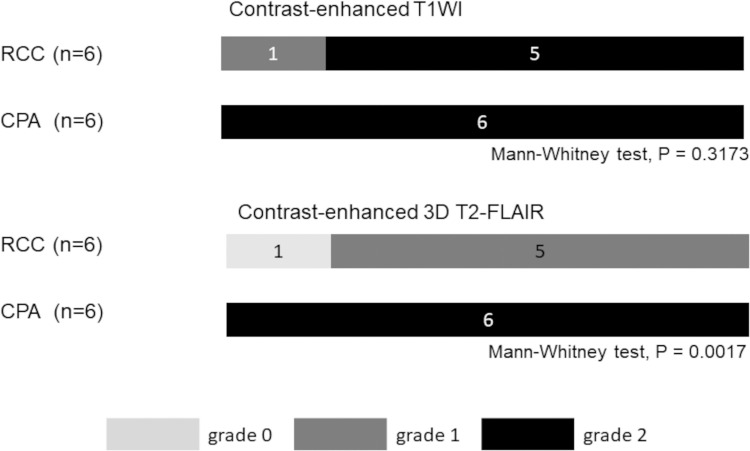
Interobserver agreement for post-contrast T1W- and 3D T2-FLAIR images was excellent (κ = 1.000 and κ = 0.885, respectively). A summary of the cyst enhancement grades of the six RCCs and four CPAs on CE-T1W- and CE-3D T2-FLAIR images is shown in Fig. 1. On CE-T1W images, all CPAs and five RCCs were classified as grade 2. There was no significant difference of the mean grade between CPAs and RCCs. On CE-3D T2-FLAIR images, all CPAs were classified as grade 2 (Fig. 2). On the other hand, four RCCs was classified as grade 0 (Fig. 3) and the others as grade 1. The difference in the mean grade assigned to CPAs and RCCs was significant (Mann–Whitney U-test, P = 0.0017). Of the six CPAs, three CPAs (50%) showed remarkable, donut-like enhancement along the inner margin of the cyst on CE-3D T2-FLAIR images (Fig. 2).
Fig. 1.
Distribution of the wall enhancement grade of a RCC and a CPA on contrast-enhanced T1W- and 3D T2-FLAIR images.
Grade 2: most of the cyst wall and/or cyst inner margin enhanced; grade 1, some of the cyst wall and/or cyst inner margin enhanced; grade 0, no enhancement in the cyst wall and cyst inner margin. 3D T2-FLAIR, 3D T2 fluid-attenuated inversion-recovery; CPA, cystic pituitary adenoma; RCC, Rathke’s cleft cyst; T1W, T1-weighted.
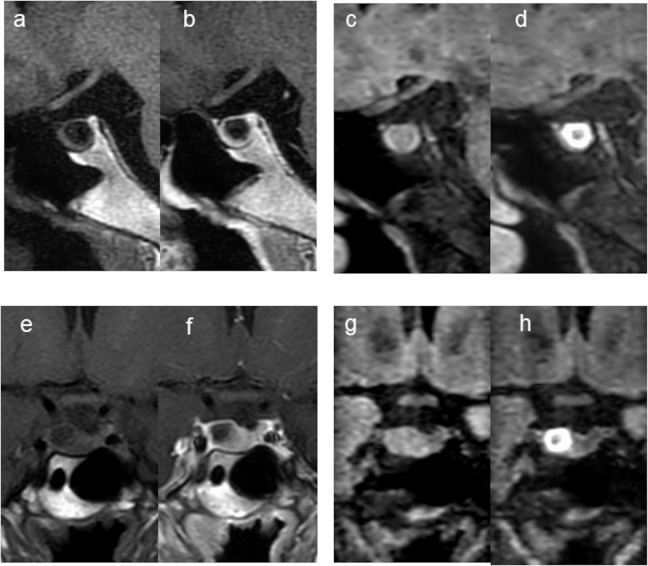
Fig. 2.
A 39-year-old woman with CPA (Case 8). Compared with the pre-contrast sagittal and coronal T1W images (a and e), the post-contrast sagittal and coronal T1W images (b and f) show cyst wall enhancement (grade 2). Pre- and post-contrast 3D T2-FLAIR sagittal (c and d) and coronal images (g and h). The post-contrast sagittal and coronal images (d and h) show remarkable, donut-like enhancement along the inner margin of the cyst (grade 2). The 3rd observer judged this lesion as an equivocal CPA (scale 3) at the 1st session but changed the confidence level to scale 5 at the 2nd session. 3D T2-FLAIR, 3D T2 fluid-attenuated inversion-recovery; CPA, cystic pituitary adenoma; T1W, T1-weighted.
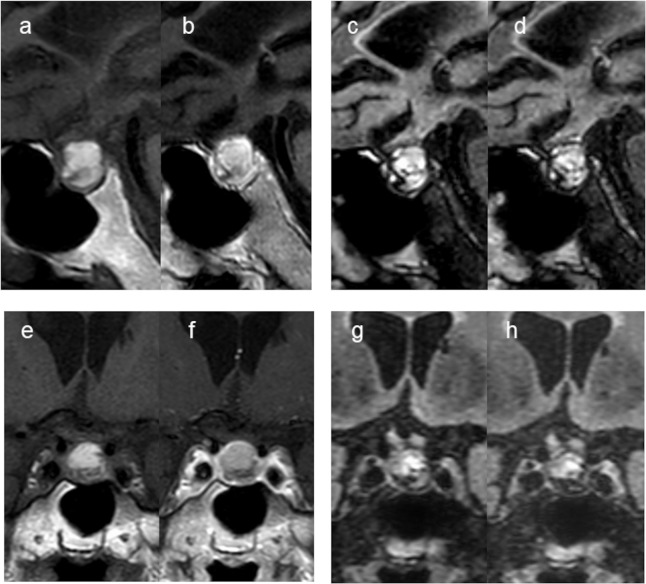
Fig. 3.
An 80-year-old man with RCC (Case 1). The pre-contrast sagittal and coronal T1W images (a and e) show high signal in the cystic lesion. The post-contrast sagittal and coronal T1W images (b and f) show wall enhancement (grade 2). Compared with the pre-contrast 3D T2-FLAIR sagittal and coronal images (c and g), there is no wall enhancement on the post-contrast 3D T2-FLAIR sagittal and coronal images (d and h) (grade 0). The 3rd observer judged this lesion as a probable CPA (scale 4) at the 1st session but changed the assessment to probable RCC (scale 2) at the 2nd session. 3D T2-FLAIR, 3D T2 fluid-attenuated inversion-recovery; CPA, cystic pituitary adenoma; RCC, Rathke’s cleft cyst; T1W, T1-weighted.
The diagnostic scales for each case on the 1st and 2nd interpretation session are shown in Table 1. The sensitivity, specificity, and accuracy in the 1st session were 1.00, 0.50, and 0.75, respectively, and improved to 1.00, 0.83, and 0.92 in the 2nd session. A retrospective review showed that 3 of 12 lesions (25%) were incorrectly interpreted in the 1st session. For two (67%) of the three misinterpreted lesions, the second interpretation led to a correct diagnosis. In one CPA patient, the lesion determination was equivocal on conventional MR scans because the enhanced wall was relatively thin on CE-T1W images (Fig. 2). In one RCC patient, the lesion determination on conventional MR scans was false-positive due to relatively thick-wall enhancement on CE-T1WI images (Fig. 3). In both cases, a correct interpretation was obtained when 3D T2-FLAIR images were added.
Table 1.
Diagnostic scales of each case in the 1st and 2nd interpretation session.
| Lesion type | Case | 1st session | 2nd session |
|---|---|---|---|
| RCC | 1 | 4 | 2 |
| 2 | 1 | 1 | |
| 3 | 3 | 2 | |
| 4 | 4 | 3 | |
| 5 | 2 | 1 | |
| 6 | 2 | 1 | |
| CPA | 7 | 4 | 5 |
| 8 | 3 | 5 | |
| 9 | 3 | 5 | |
| 10 | 3 | 5 | |
| 11 | 5 | 5 | |
| 12 | 5 | 5 |
1 = definitely RCC, 2 = probably RCC, RCC3 = equivocal, 4 = probably CPA, 5 = definitely CPA. A lesion with a confidence score of 1 or 2 was considered as RCC; those with a score of 3, 4, or 5 as CPA. CPA, cystic pituitary adenoma; RCC, Rathke’s cleft cyst.
Discussion
Our study showed that CPAs and RCCs differed significantly with respect to their cyst wall enhancement on CE 3D T2-FLAIR images and that adding CE 3D T2-FLAIR images to conventional MR images was useful for their differentiation. To our knowledge, ours is the first documentation that CE 3D T2-FLAIR imaging is useful for the differentiation between an RCCs and CPAs.
We think that the differences in the cyst wall enhancement of CPAs and RCCs on CE 3D T2-FLAIR images can be explained as follows: In CPAs, the cyst wall is composed of tumor cells, while it is comprised of the normal anterior pituitary gland in RCCs.21 On CE 3D T2-FLAIR images, the anterior lobe of the pituitary gland is often unenhanced;24 consequently, the wall of RCCs is usually not enhanced.23 This may be attributable to T2 shortening due to a high gadolinium concentration or to the sequence’s sensitivity to flow in the hypophyseal portal system, which, in the anterior pituitary gland, is composed of sinusoidal capillaries.24 On the other hand, the wall of CPAs on CE 3D T2-FLAIR images does enhance, probably due to the vessel permeability of these neoplasms. Therefore, CE 3D T2-FLAIR imaging reflects differences in the composition of the wall of CPAs and RCCs.
On CE 3D T2-FLAIR images, three CPAs (50%) showed remarkable, donut-like enhancement along the inner margin of the cyst (Fig. 2); this was not the case on CE-T1W images. It has been reported that the incidence of spontaneous infarction of pituitary adenomas is high and that the infarction of adenomas may or may not be accompanied by hemorrhage but is followed by the formation of a cystic cavity.25–28 As the infarction of pituitary adenomas may result in increased vascular permeability in the infarct region, the contrast agent may leak into the cyst fluid through the tumor vessels at the infarct site. Since the CE 3D T2-FLAIR is more sensitive to low gadolinium concentrations than the T1W sequence,22 gadolinium leaked in the cystic fluid of CPAs may be observed on CE 3D T2-FLAIR images. In a previous study,23 donut-like enhancement along the inner margin of the cystic wall of craniopharyngioma was not observed on CE 3D T2-FLAIR images. The donut sign, donut-like enhancement along the inner margin of the cyst on CE 3D T2-FLAIR images, may be useful for discriminating not only CPA and RCC but also CPA and cystic craniopharyngioma.
Park et al.17 reported that a combination of imaging characteristics (an off-midline location, a fluid-fluid level or a hypointense rim on T2W MR images, septation, and an intracystic nodule) returned a high AUC value (0.991) for the differentiation between the two types of lesions. We found that adding CE 3D T2-FLAIR to conventional MR images raised the sensitivity, specificity, and accuracy from 1.00, 0.50, and 0.75 to 1.00, 0.83, and 0.92, respectively.
Our study has some limitations. It was a single-center, retrospective investigation and the study population was small. We did not do quantitative analysis in this study. A region of interest could not be set in the cyst wall of the lesion due to the thinness of the cyst wall. Also, the time-dependence of gadolinium enhancement may have affected our results. The first CE sequence we acquired in all patients was the conventional T1W sequence, and because the 3D sequence was subsequently obtained, we might have overestimated the added value of the 3D sequence. In addition, we did not compare CE 3D T2-FLAIR imaging with dynamic contrast-enhanced imaging (e.g., Ktrans maps).29 Further studies with the two imaging sequences may clarify the relationship between the degree of enhancement on CE 3D T2-FLAIR images and the permeability on Ktrans maps.
Conclusion
On CE 3D T2-FLAIR images, three CPAs (50%) showed remarkable, donut-like enhancement along the inner margin of the cyst; this finding was not seen on CE-T1W images. Consequently, we suggest that CE 3D T2-FLAIR images are useful for differentiating between CPAs and RCCs. Donut-like enhancement along the inner margin of the cyst on CE 3D T2-FLAIR images can be a feature sign of CPA.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Goel A, Shah A, Jhawar SS, et al. Fluid-fluid level in pituitary tumors: analysis of management of 106 cases. J Neurosurg 2010; 112:1341–1346. [DOI] [PubMed] [Google Scholar]
- 2.Chacko AG, Chacko G, Seshadri MS, et al. Hemorrhagic necrosis of pituitary adenomas. Neurol India 2002; 50:490–493. [PubMed] [Google Scholar]
- 3.Semple PL, Jane JA, Lopes MB, et al. Pituitary apoplexy: correlation between magnetic resonance imaging and histopathological results. J Neurosurg 2008; 108:909–915. [DOI] [PubMed] [Google Scholar]
- 4.Park CK, Kim DC, Park SH, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg 2006; 105:576–580. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zhang W, Fu LA, et al. Hemorrhagic pituitary macroadenoma: characteristics, endoscopic endonasal transsphenoidal surgery, and outcomes. Ann Surg Oncol 2011; 18:246–252. [DOI] [PubMed] [Google Scholar]
- 6.Semple PL, Webb MK, de Villiers JC, et al. Pituitary apoplexy. Neurosurgery 2005; 56:65–72. discussion 72–73. [DOI] [PubMed] [Google Scholar]
- 7.Piotin M, Tampieri D, Ruefenacht DA, et al. The various MRI patterns of pituitary apoplexy. Eur Radio 1999; 9:918–923. [DOI] [PubMed] [Google Scholar]
- 8.Tosaka M, Sato N, Hirato J, et al. Assessment of hemorrhage in pituitary macroadenoma by T2*-weighted gradient-echo MR imaging. AJNR Am J Neuroradiol 2007; 28:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurihara N, Takahashi S, Higano S, et al. Hemorrhage in pituitary adenoma: correlation of MR imaging with operative findings. Eur Radiol 1998; 8:971–976. [DOI] [PubMed] [Google Scholar]
- 10.Rennert J, Doerfler A. Imaging of sellar and parasellar lesions. Clin Neurol Neurosurg 2007; 109:111–124. [DOI] [PubMed] [Google Scholar]
- 11.Ross DA, Norman D, Wilson CB. Radiologic characteristics and results of surgical management of Rathke’s cysts in 43 patients. Neurosurgery 1992; 30:173–178. discussion 178-179. [DOI] [PubMed] [Google Scholar]
- 12.Sumida M, Uozumi T, Mukada K, et al. Rathke cleft cysts: correlation of enhanced MR and surgical findings. AJNR Am J Neuroradiol 1994; 15:525–532. [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta GU, Jane JA. Pituitary tumors. Curr Opin Neurol 2012; 25:751–755. [DOI] [PubMed] [Google Scholar]
- 14.Nishioka H, Haraoka J, Izawa H, et al. Magnetic resonance imaging, clinical manifestations, and management of Rathke’s cleft cyst. Clin Endocrinol (Oxf) 2006; 64:184–188. [DOI] [PubMed] [Google Scholar]
- 15.Buchfelder M, Schlaffer S. Surgical treatment of pituitary tumours. Best Pract Res Clin Endocrinol Metab 2009; 23:677–692. [DOI] [PubMed] [Google Scholar]
- 16.Han SJ, Rolston JD, Jahangiri A, et al. Rathke’s cleft cysts: review of natural history and surgical outcomes. J Neurooncol 2014; 117:197–203. [DOI] [PubMed] [Google Scholar]
- 17.Park M, Lee SK, Choi J, et al. Differentiation between cystic pituitary adenomas and Rathke cleft cysts: a diagnostic model using MRI. AJNR Am J Neuroradiol 2015; 36:1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SH, Kwon BJ, Na DG, et al. Pituitary adenoma, craniopharyngioma, and Rathke cleft cyst involving both intrasellar and suprasellar regions: differentiation using MRI. Clin Radiol 2007; 62:453–462. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Hu LB, Feng XY, et al. Rathke’s cleft cyst: clinicopathological and MRI findings in 22 patients. Clin Radiol 2010; 65:47–55. [DOI] [PubMed] [Google Scholar]
- 20.Hua F, Asato R, Miki Y, et al. Differentiation of suprasellar nonneoplastic cysts from cystic neoplasms by Gd-DTPA MRI. J Comput Assist Tomogr 1992; 16:744–749. [DOI] [PubMed] [Google Scholar]
- 21.Byun WM, Kim OL, Kim D. MR imaging findings of Rathke’s cleft cysts: significance of intracystic nodules. AJNR Am J Neuroradiol 2000; 21:485–488. [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuoka H, Hirai T, Okuda T, et al. Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization-prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomeningeal diseases at 3T. AJNR Am J Neuroradiol 2010; 31:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma M, Khant ZA, Kitajima M, et al. Usefulness of contrast-enhanced 3D-FLAIR MR imaging for differentiating Rathke cleft cyst from cystic craniopharyngioma. AJNR Am J Neuroradiol 2020; 41:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma M, Hirai T, Kadota Y, et al. Circumventricular organs of human brain visualized on post-contrast 3D fluid-attenuated inversion recovery imaging. Neuroradiology 2018; 60:583–590. [DOI] [PubMed] [Google Scholar]
- 25.Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg 2015; 122:1444–1449. [DOI] [PubMed] [Google Scholar]
- 26.Liu JK, Couldwell WT. Pituitary apoplexy in the magnetic resonance imaging era: clinical significance of sphenoid sinus mucosal thickening. J Neurosurg 2006; 104:892–898. [DOI] [PubMed] [Google Scholar]
- 27.De Villiers JC, Marcus G. Non-hemorrhagic infarction of pituitary tumors presenting as pituitary apoplexy. Adv Biosci 1988; 69:461–464. [Google Scholar]
- 28.Ostrov SG, Quencer RM, Hoffman JC, et al. Hemorrhage within pituitary adenomas: how often associated with pituitary apoplexy syndrome? AJR Am J Roentgenol 1989; 153:153–160. [DOI] [PubMed] [Google Scholar]
- 29.Kamimura K, Nakajo M, Yoneyama T, et al. Quantitative pharmacokinetic analysis of high-temporal-resolution dynamic contrast-enhanced MRI to differentiate the normal-appearing pituitary gland from pituitary macroadenoma. Jpn J Radiol 2020; 38:649–657. [DOI] [PubMed] [Google Scholar]