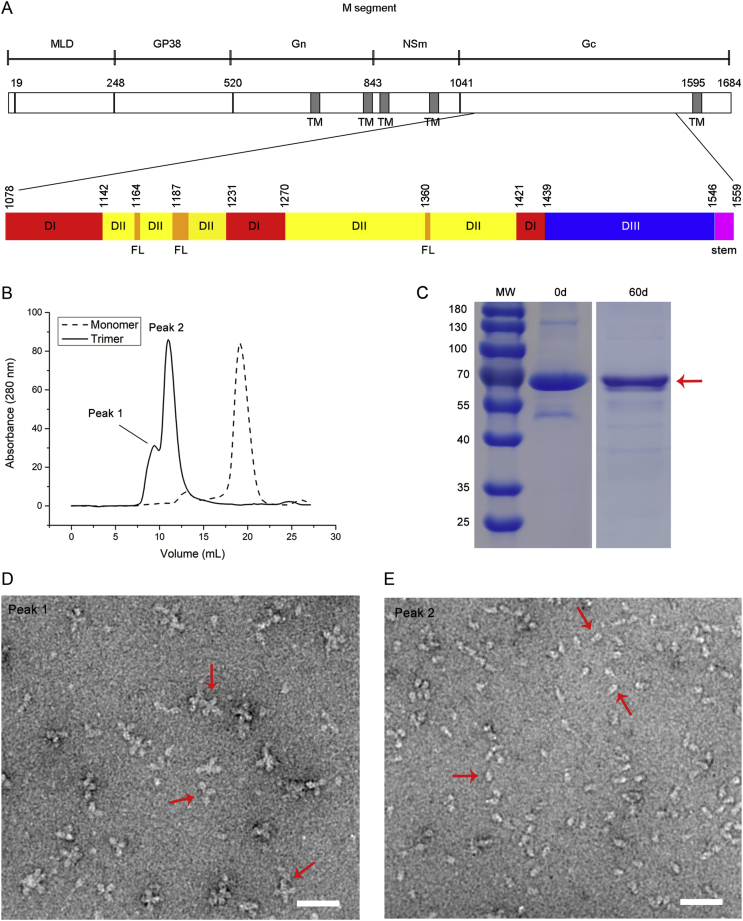
Fig. 1.
Expression of CCHFV sGc-trimers. A Domain diagram of the CCHFV M RNA segment encoding MLD, GP38, Gn, NSm and Gc and transmembrane (TM) regions, which are shown in dark gray. The first residue of each mature protein is provided above. The lower schematic outlines the ectodomain organization of Gc based on our cryo-EM structure: domain I (DI) in red, domain II (DII) in yellow with the fusion loop (FL) in orange, domain III (DIII) in blue and the stem region in magenta. The first residue of each domain is provided above. B SEC elution profiles of CCHFV Gc monomer and trimer are shown as dashed and solid lines, respectively. There are two oligomeric states for sGc-trimers, as indicated by peaks 1 and 2. C SDS-PAGE analysis of degradation of purified sGc-trimers (marked by a red arrow) at 4 °C after 60 days. D and E Negative staining analysis of purified sGc-trimers in peaks 1 and 2 from Fig. 1B. Red arrows indicate rosette-shaped particles formed by aggregation of sGc-trimers (D) and cone-shaped sGc-trimers (E). Scale bar: 50 nm. MLD, mucin-like domain; SEC, size-exclusion chromatography; CCHFV, Crimean-Congo hemorrhagic fever virus; sGc-trimer, trimer of the soluble Gc ectodomain.