Abstract
Morphea also called localized scleroderma is a complex entity that requires objective methods for supporting the diagnosis, severity, and activity. To date, clinical scorings may show a very good inter-rater agreement but cannot provide anatomical information on subclinical involvement. Biological markers can be used for detecting inflammation but may not be useful for grading tissue damage. Color Doppler ultrasound can support diagnosis and the assessment of severity and activity in morphea which has been validated using histology as the gold standard. Ultrasound is the first-choice imaging technique for studying cutaneous diseases and can show subclinical involvement, including the affection of deeper layers non-invasively and safely. It requires proper ultrasound devices, imaging-trained physicians for performing the examinations, the performance of a standardized protocol during the examinations, and an organized schedule that allows enough time for evaluating the patients. Under the latter conditions, ultrasonography can be a powerful and reliable tool for supporting the management of morphea.
Keywords: Morphea, morphea ultrasound, scleroderma ultrasound, dermatologic ultrasound, skin ultrasound
Introduction
Morphea also called localized scleroderma is a chronic autoimmune disorder of the connective tissue that produces inflammation and fibrosis of the skin and under-lying soft tissues.1,2 Its clinical diagnosis may be challenging, complex, and sometimes delayed.3,4 The monitoring of activity of morphea only-based on clinical and laboratory parameters can be less accurate because they do not provide anatomical information on severity or activity. This fact is critical because morphea tend to affect young individuals in highly exposed areas of the body such as the face and may produce disfigurement; 5 therefore, a decrease of the self-esteem and quality of life may be easily generated.4,6
The clinical appearance of morphea lesions can be deceiving because as reported on ultrasound imaging, hypodermal inflammation can be subclinical and neither produce erythema nor induration. 7 Lesions presenting cutaneous atrophy or hyperpigmentation can be at the same time active in the center or the borders. 8
Histological diagnosis is not exempted from difficulties because it relies on the size of the sample and the location of the biopsy. If the sample was too superficial and did not include enough hypodermis, or was acquired in the wrong place, the diagnosis is limited. 9 Besides, the monitoring of morphea with biopsies can leave scars, besides being a potential trigger for more inflammation and fibrosis. In addition, histological samples may not show subclinical sites of involvement. Furthermore, in routine clinical practice, the biopsy is not performed in a significant number of cases, and the monitoring of the patient relies on the clinical signs.
Even though the clinical signs are of paramount importance for diagnosing morphea, the use of clinical scorings can be time-consuming and difficult to standardize. For example, the clinical discrimination of a cutaneous moderate or severe thickness may be sensitive 10 but not necessarily indicate severity or activity. Moreover, in a recently published series, the localized scleroderma skin damage index (LoSDI) was qualified as unreliable for predicting damage of the tissues. 11 On the other hand, the assessment of a good inter-rater agreement coefficient in a scoring system may be not considered as the equivalent of a perfect detection of severity or activity; particularly, if there is no clear gold standard for comparison such as histology or an imaging technique. 12
Thus, to date, neither clinical scorings nor biological markers can simultaneously assess activity and degree of tissue damage. This issue implies that morphea can lack evidence-based therapies due to the absence of reliable outcome measurements and long-term monitoring of the treatments.
Imaging modalities for evaluating morphea
Several imaging modalities have been tested for supporting the diagnosis and monitoring in morphea. For example, thermography has been used for evaluating activity; however, it requires a temperature-controlled room and presents difficulties for measuring scalp and facial lesions. Besides, to increase the sensitivity of this technique, some authors have suggested the usage of non-arbitrary and more precise cut-off values. 13
Magnetic resonance imaging (MRI) has also been explored in small series for detecting thickening of the dermal and fascial layers in morphea or ruling out brain involvement.14,15 Nevertheless, this high-cost imaging modality presents lower resolution for detecting cutaneous and ungual alterations. 16 In addition, MRI requires the intravenous injection of contrast medium which by itself can be a potential trigger for fibrotic disorders. 17 Thus, in routine clinical practice, thermography or MRI is rarely used for diagnosing or monitoring morphea patients.
Usage of color Doppler ultrasound in morphea
Color Doppler ultrasound has been reported as a reliable tool for monitoring the activity of morphea using variable and high-frequency ultrasound probes that vary in their upper range between 14 and 18 MHz.18–23 This imaging modality has also supported the characterization of cutaneous ulcers in scleroderma patients. 24 Recent ultrasound guidelines recommend the use of probes ⩾15 MHz for performing dermatologic ultrasound studies and discourage the exclusive usage of equipment with very high-frequency probes (⩾30 MHz) due to low of penetration. 25 For that reason, in morphea or others inflammatory cutaneous diseases, the performance of ultrasound examinations with very high-frequency probes cannot detect structural alterations or hypervascularity in the dermal-hypodermal junction, hypodermis, fascial or muscular layers. The latter is a relevant point because the use of high resolution and very high-frequency probes is not an analogy of having better definition or detection of alterations, particularly in conditions where the abnormalities are mainly located in the lower dermis, hypodermis, or deeper layers. 26 In the past, the great variety of very-high and high-frequency ultrasound machines used in dermatology, particularly under research settings and by non-trained operators has confused the proper selection of sonographic devices for studying the skin, defining and comparing the most characteristic ultrasound patterns, and assessing standardized protocols of study. 27
An improvement of the sonographic detection of abnormalities in the ultrasound examinations can be achieved through the follow-up of the published guidelines for performing dermatologic ultrasound studies and the conjoined work of a multispecialty medical team that could include imaging-trained physicians.8,25 As in any other field of medicine, the performance of sonographic examinations by non-imaging-trained physicians can decrease the sensitivity of the ultrasound detection. The latter situation also happens in other imaging techniques such as MRI because if the protocol is not well selected, the alterations may be not detected.
On ultrasonography, the live interaction between the patient and the physician in charge of the examination can support the proper selection of the corporal sections and the detection of the affected layers. 16 Thus, a clinically non-evident site of abnormality or activity in morphea may be easier to find and track.
Ultrasound can provide qualitative and quantitative anatomical data in morphea such as measurements of the thickness, detection of structural abnormalities, use of anatomical landmarks, and the possibility of grading the inflammation through the usage of color Doppler and spectral curve analysis. 7 This information includes the assessment of the amount, type (arterial or venous), and velocity of the vessels (cm/s) in the lesional and perilesional areas. All these measurements can provide more objective criteria for evaluating treatment response in a clinical or research setting, including trials. Moreover, the sonographic signs used for supporting the diagnosis and monitoring morphea have correlated very well with the histological findings and showed very high sensitivity. 20 In morphea, ultrasound has also been used for assessing the therapeutic response to phototherapy, 23 guide hydrodissection and corticosteroid injection 28 as well as to select the biopsy site. 8,12
Color Doppler ultrasound for supporting diagnosis and detecting activity in morphea
The sonographic appearance of morphea will depend on the form of presentation and the phase of the lesions. Therefore, on sonography, active lesions in the inflammatory stage will present a different morphology in comparison with atrophic and inactive lesions. In addition, concomitant morpheiform conditions can be present in the same patient and sometimes, these are not evident in the physical examination. 20 For example, a patient with a lichen sclerosus can show sonographic signs of deeper hypodermal involvement in the same or a different corporal site, which may be difficult to deduct from the physical examination. On the other hand, a patient with eosinophilic fasciitis can present subclinical deep dermal or hypodermal signs of inflammation.
Color Doppler ultrasound signs of morphea
There are ultrasound signs that can support the diagnosis and the assessment of activity and severity of morphea. During the active inflammatory phase, the lesions will tend to present loss of definition of the dermal-hypodermal border and a diffuse or partial increase of echogenicity of the hypodermis. Dermal and hypodermal hypervascularity can also be detected. The presence of thickening and decreased echogenicity of the dermis may be found on morphea, but these are not considered signs of activity due to their low specificity. The reason is that these dermal alterations can be seen in other types of chronic inflammatory conditions, besides morphea.
In some cases, sonographic signs of mixed or septal panniculitis can be detected such as hyperechogenicity of the fat lobules as well as thickening and hypoechogenicity of the hypodermal septa. In Parry–Romberg morphea (morphea with hemifacial atrophy), concomitant signs of chronic inflammation of the parotid gland of the affected side can be detected. These include decreased echogenicity and size of the parotid gland and occasionally, the presence of glandular hypervascularity.7,20
In the inactive atrophic phase, there is thinning of the dermis and hypodermis that commonly show areas that do not contain hypodermal fatty tissue which can produce a close contact between the dermis and the fascial layer. At the end stage of atrophy, the dermis and hypodermis can show increased echogenicity with a fibrillar pattern due to the prominent presence of collagen fibers and lack of fatty tissue.7,20
The most sensitive ultrasound signs for detecting activity in morphea that have been reported are the detection of dermal and/or hypodermal hypervascularity and the increase of hypodermal echogenicity. 20 A relevant point to consider is that the presence of clinical signs of atrophy and hyperpigmentation does not necessarily mean inactivity because morphea plaques may still show sonographic signs of activity in the center and/or the borders. 7
In addition, patients with several lesions may present an asynchronous activity of the plaques on the ultrasound examination. Thus, some of the cutaneous plaques may be sonographically active and others inactive 7 (Figures 1–8).
Figure 1.
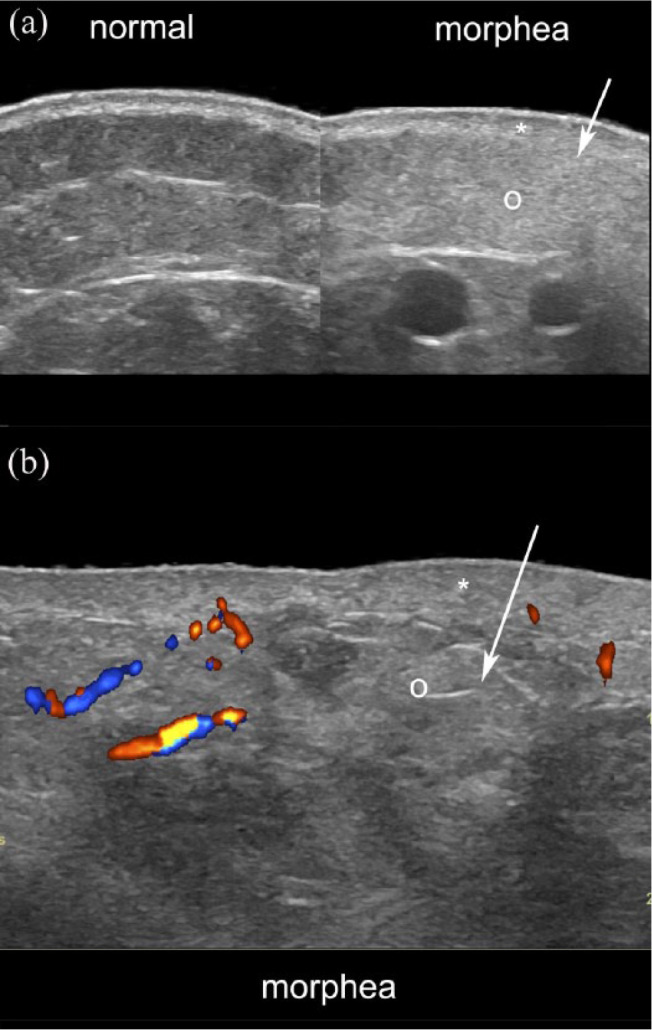
Ultrasound signs of active morphea. (a) Greyscale ultrasound (side-by-side comparison of normal perilesional with the lesional site) and (b) color Doppler present dermal thickening (*), loss of definition of the dermal and hypodermal border (arrows), increased echogenicity of the hypodermis (o) and increased dermal and hypodermal vascularity (b, in colors).
Figure 2.
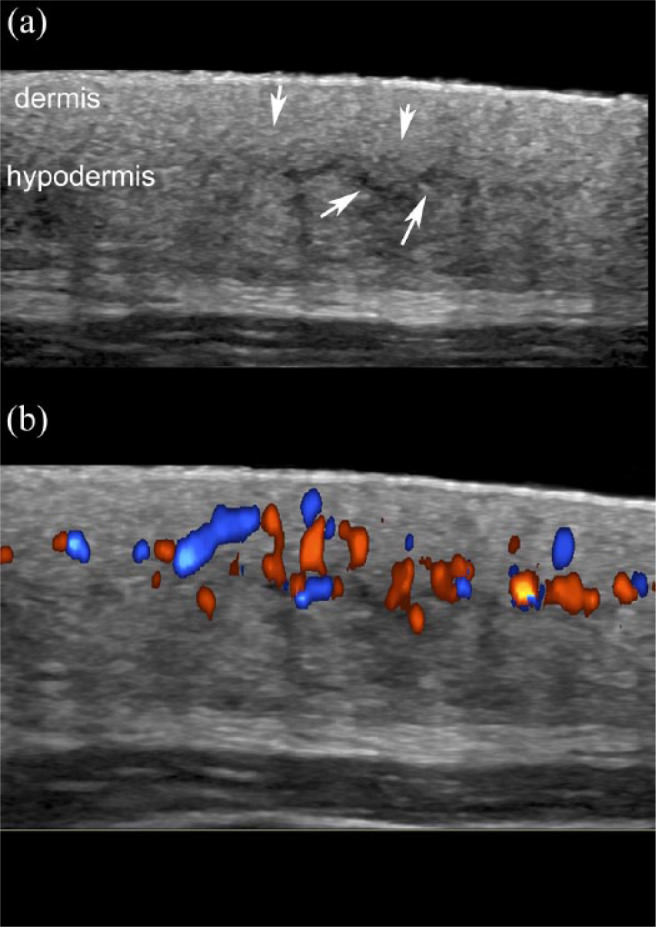
Active morphea with signs of septal panniculitis. (a) Greyscale and (b) color Doppler demonstrate loss of the dermal and hypodermal border (arrows pointing down), increased echogenicity of the hypodermis and thickening with decreased echogenicity of the fibrous hypodermal septa (arrows pointing up in (a)). Note the increased dermal and hypodermal vascularity in (b).
Figure 3.
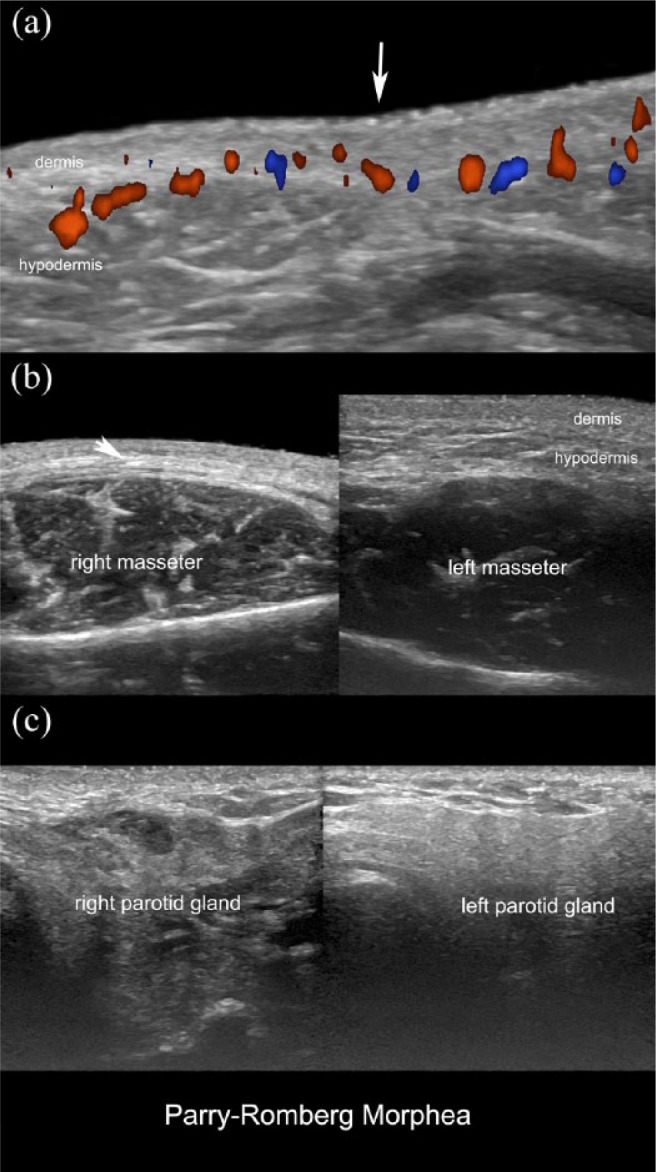
Active Parry–Romberg morphea with facial hemiatrophy of the right side. (a) Color Doppler ultrasound (transverse view; right peribuccal region) demonstrates region (arrow pointing down) with decreased thickness of the dermis and hypodermis, loss of the fatty tissue of the hypodermis, and increased dermal and hypodermal vascularity. (b) Greyscale (side-by-side transverse view of the cheeks; right lesional) demonstrates dermal and hypodermal thinning (oblique arrow), loss of the hypodermal fatty tissue, and increased echogenicity of the hypodermis of the right side. (c) Greyscale ultrasound (side-by-side transverse views of the preauricular regions) shows decreased echogenicity of the right parotid gland due to chronic inflammation.
Figure 4.
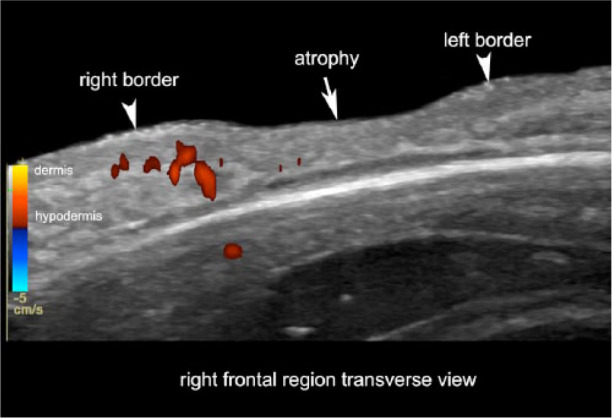
Active morphea in the borders of an atrophy site. Color Doppler ultrasound (transverse view; right frontal region) presents signs of activity predominantly at the right border of a frontal lesion (left side of the image). Note the loss of definition of the dermal and hypodermal borders and the increased vascularity (in colors) at the right border.
Figure 5.
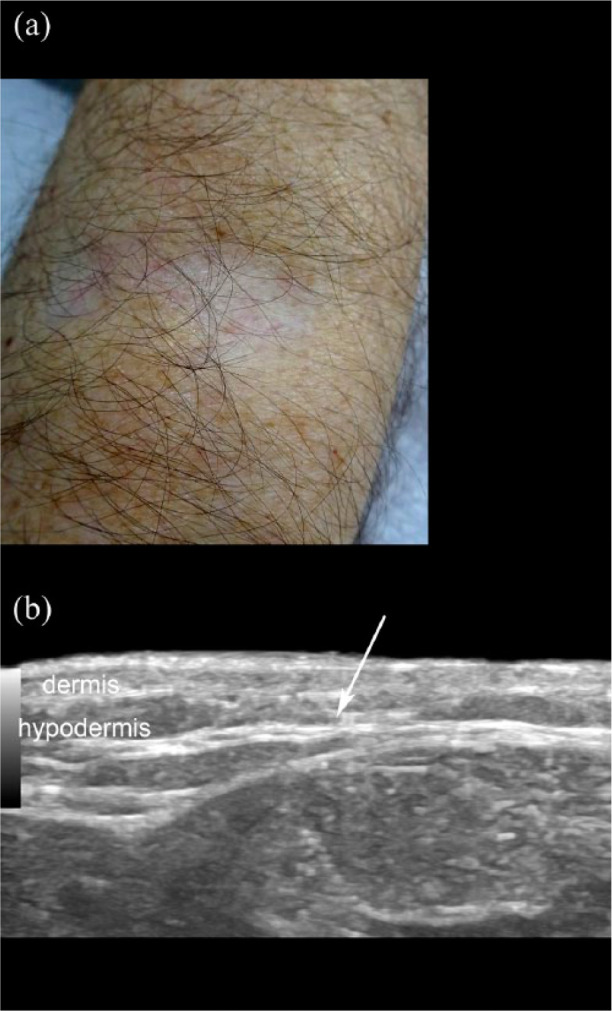
Active morphea and lichen sclerosus. (a) Clinical image of the right forearm of a patient with lichen sclerosus. (b) Ultrasound (transverse view; right forearm) of the clinical lesion area presents a loss of the dermal and hypodermal border with increased echogenicity of the hypodermis (arrow).
Figure 6.
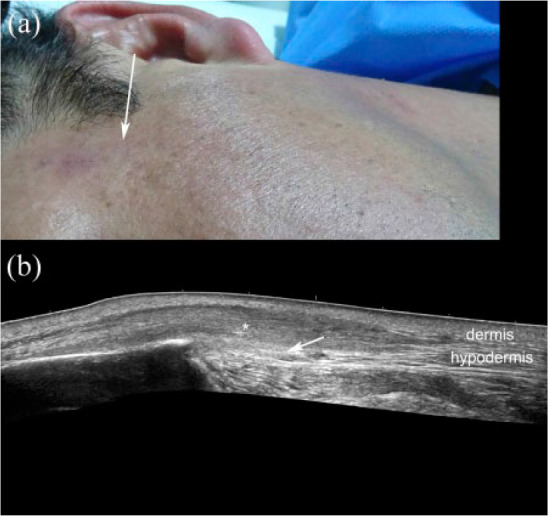
Morphea with eosinophilic fasciitis. (a) Clinical photograph of the lesion. (b) Ultrasound (gray scale; transverse view; left temple region) demonstrates a hypoechoic area with a fibrillar pattern (*) in the hypodermis that reaches the fascial layer (arrow). Note the increased echogenicity of the hypodermis at the anterior border of the temple (left side of the image).
Figure 7.
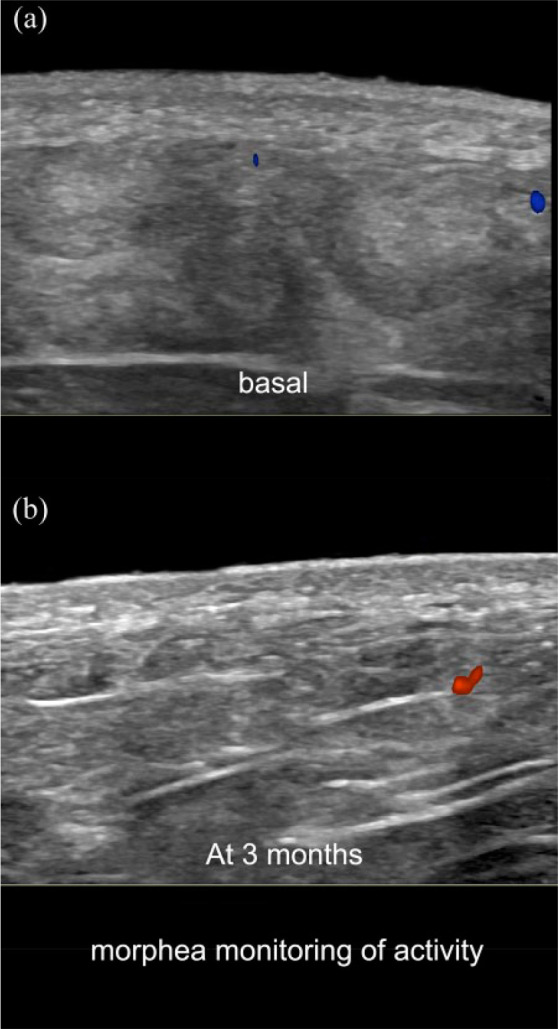
Color Doppler ultrasound monitoring of morphea (transverse views; left gluteal region). (a) Basal examination with signs of activity (loss of the dermal and hypodermal border and increased hypodermal echogenicity). (b) Follow-up ultrasound at 3 months shows improvement in the definition of the border and decrease of the abnormal echogenicity of the hypodermis. Note the hypoechogenicity of the dermis in both images which is more prominent in the basal examination.
Figure 8.
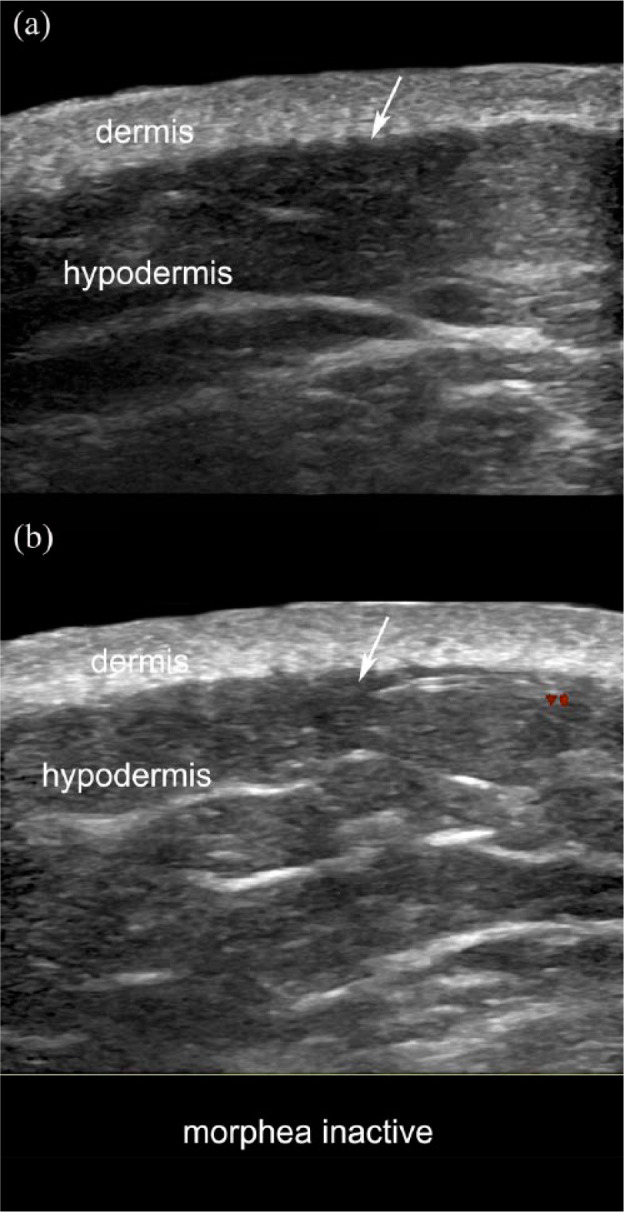
Inactive morphea. (a) Greyscale and (b) color Doppler (transverse views; right arm) show a slight dermal thickening with decreased dermal echogenicity. Note that the dermal-hypodermal border is well-defined and there are no signs of increased echogenicity of the hypodermis or hypervascularity (arrows). Prominent fibrous septa in the hypodermis are also detected.
How to scan morphea
Considering the availability of adequate equipment, a trained imaging physician, and the performance of a dermatologic protocol for studying dermatologic lesions, a topographic color Doppler ultrasound of the affected body region(s) is recommended. This sonographic study includes an ultrasound sweep in at least two perpendicular axes of the corporal segment and not just the plaque area. Usually, the contralateral and adjacent anatomical regions are also scanned to rule out the presence of subclinical involvement. For example, color Doppler ultrasound examinations of linear morphea (“coup de sabre”), a subtype that usually affects the frontal region of the face, should also include a sonographic sweep of the scalp, following the same axis of the frontal facial lesion; otherwise, part of the alterations and activity can be missed.18,20 Comparison with the normal contralateral and perilesional skin can help; however, the sonographic signs of abnormalities should not rely solely on comparisons because these sites may also be abnormal.
It should be kept in mind that it is necessary to assign enough time for scanning the patients and reporting the abnormalities; therefore, the inclusion of a schedule of patients that consider proper time for the color Doppler ultrasound examinations is mandatory to get adequate results. Patients with involvement of one corporal segment may be scanned in 20 or 30 min; however, patients with the affection of multiple body sites may need 1 h or more depending on the complexity of the case and the experience of the operator.
Nowadays, morphea is still a challenging entity 29 that presents gaps in the diagnosis and management. So far, there are advances, and the usage of ultrasonography has been mentioned in some of the recently published guidelines for managing this disease that consider the activity of the disease as a crucial factor for selecting the treatment. 30 The consideration of imaging techniques, particularly, color Doppler ultrasound, in the routine evaluation of morphea may significantly improve the precision of the diagnosis and management as well as the evidence of the response to treatments in these patients.
In conclusion, color Doppler ultrasound can be a reliable imaging modality for supporting the diagnosis and assessing the severity and activity of morphea. The usage of adequate devices, the availability of an imaging-trained physician, and the performance of a standardized protocol are needed for improving the precision in the diagnosis, management, and research of morphea.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ximena Wortsman  https://orcid.org/0000-0003-3359-5023
https://orcid.org/0000-0003-3359-5023
References
- 1. Fett N, Werth VP. Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol 2011; 64: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sartori-Valinotti JC, Tollefson MM, Reed AM. Updates on morphea: role of vascular injury and advances in treatment. Autoimmune Dis 2013; 2013: 467808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol 2015; 90: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol 2011; 165: 1308–1313. [DOI] [PubMed] [Google Scholar]
- 5. Mertens JS, Seyger MMB, Thurlings RM, et al. Morphea and eosinophilic fasciitis: an update. Am J Clin Dermatol 2017; 18: 491–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bali G, Kárpáti S, Sárdy M, et al. Association between quality of life and clinical characteristics in patients with morphea. Qual Life Res. Epub ahead of print 19 June 2018. DOI: 10.1007/s11136-018-1897-1 [DOI] [PubMed] [Google Scholar]
- 7. Wortsman X, Carreño LMC. Inflammatory diseases of the skin. In: Wortsman X, Jamec G. (eds) Dermatologic ultrasound with clinical and histologic correlations. 1st ed. New York: Springer, 2013, pp. 73–117. [Google Scholar]
- 8. Wortsman X, Gutierrez M, Saavedra T, et al. The role of ultrasound in rheumatic skin and nail lesions: a multi-specialist approach. Clin Rheumatol 2011; 30(6): 739–748. [DOI] [PubMed] [Google Scholar]
- 9. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol 2016; 74: 1–16. [DOI] [PubMed] [Google Scholar]
- 10. Arkachaisri T, Vilaiyuk S, Li S, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol 2009; 36: 2819–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agazzi A, Fadanelli G, Vittadello F, et al. Reliability of LoSCAT score for activity and tissue damage assessment in a large cohort of patients with Juvenile Localized Scleroderma. Pediatr Rheumatol 2018; 16: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bendeck SE, Jacobe HT. Ultrasound as an outcome measure to assess disease activity in disorders of skin thickening: an example of the use of radiologic techniques to assess skin disease. Dermatol Ther 2007; 20: 86–92. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Romero MT, Randhawa HK, Laxer R, et al. The role of local temperature and other clinical characteristics of localized scleroderma as markers of disease activity. Int J Dermatol 2017; 56: 63–67. [DOI] [PubMed] [Google Scholar]
- 14. Eutsler EP, Horton DB, Epelman M, et al. Musculoskeletal MRI findings of juvenile localized scleroderma. Pediatr Radiol 2017; 47: 442–449. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi T, Asano Y, Oka T, et al. Scleroderma en coup de sabre with recurrent episodes of brain hemorrhage. J Dermatol 2016; 43: 203–206. [DOI] [PubMed] [Google Scholar]
- 16. Wortsman X. Common applications of dermatologic sonography. J Ultrasound Med 2012; 31: 97–111. [DOI] [PubMed] [Google Scholar]
- 17. Pasquini L, Napolitano A, Visconti E, et al. Gadolinium-based contrast agent-related toxicities. CNS Drugs 2018; 32: 229–240. [DOI] [PubMed] [Google Scholar]
- 18. Li SC, Liebling MS, Haines KA. Ultrasonography is a sensitive tool for monitoring localized scleroderma. Rheumatology 2007; 46: 1316–1319. [DOI] [PubMed] [Google Scholar]
- 19. Li SC, Liebling MS. The use of Doppler ultrasound to evaluate lesions of localized scleroderma. Curr Rheumatol Rep 2009; 11: 205–211. [DOI] [PubMed] [Google Scholar]
- 20. Wortsman X, Wortsman J, Sazunic I, et al. Activity assessment in morphea using color Doppler ultrasound. J Am Acad Dermatol 2011; 65: 942–948. [DOI] [PubMed] [Google Scholar]
- 21. Pérez-López I, Garrido-Colmenero C, Ruiz-Villaverde R, et al. Monitorización ecográfica de la morfea lineal de la infancia. Actas Dermosifiliogr 2015; 106(4): 340–342. [DOI] [PubMed] [Google Scholar]
- 22. Wortsman X, Ma L, Chung WK, et al. Evaluation of the CAV1 gene in clinically, sonographically and histologically proven morphea patients. Exp Dermatol 2015; 24: 718–720. [DOI] [PubMed] [Google Scholar]
- 23. Buense R, Alzira I, Duarte G, et al. Localized scleroderma: assessment of the therapeutic response to phototherapy. Esclerodermia cutânea: avaliação da resposta terapêutica à fototerapia. An Bras Dermatol 2012; 87(1): 63–72. [DOI] [PubMed] [Google Scholar]
- 24. Suliman YA, Kafaja S, Fitzgerald J, et al. Ultrasound characterization of cutaneous ulcers in systemic sclerosis. Clin Rheumatol 2018; 37: 1555–1561. [DOI] [PubMed] [Google Scholar]
- 25. Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the Dermus Group. J Ultrasound Med 2016; 35: 577–580. [DOI] [PubMed] [Google Scholar]
- 26. Wortsman X. Ultrasound in dermatology: why, how, and when? Semin Ultrasound CT MR 2013; 34: 177–195. [DOI] [PubMed] [Google Scholar]
- 27. Wortsman X. Letter to the editor: the entrance echo corresponds to epidermis on high frequency skin ultrasound. J Cosmet Sci 2016; 67: 109–110. [PubMed] [Google Scholar]
- 28. DeLea SL, Chavez-Chiang NR, Poole JL, et al. Sonographically guided hydrodissection and corticosteroid injection for scleroderma hand. Clin Rheumatol 2011; 30: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valentini G, Matucci Cerinic M. Disease-specific quality indicators, guidelines and outcome measures in scleroderma. Clin Exp Rheumatol 2007; 25(6 Suppl. 47): 159–162. [PubMed] [Google Scholar]
- 30. Asano Y, Fujimoto M, Ishikawa O, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. J Dermatol 2018; 45: 755–780. [DOI] [PubMed] [Google Scholar]


