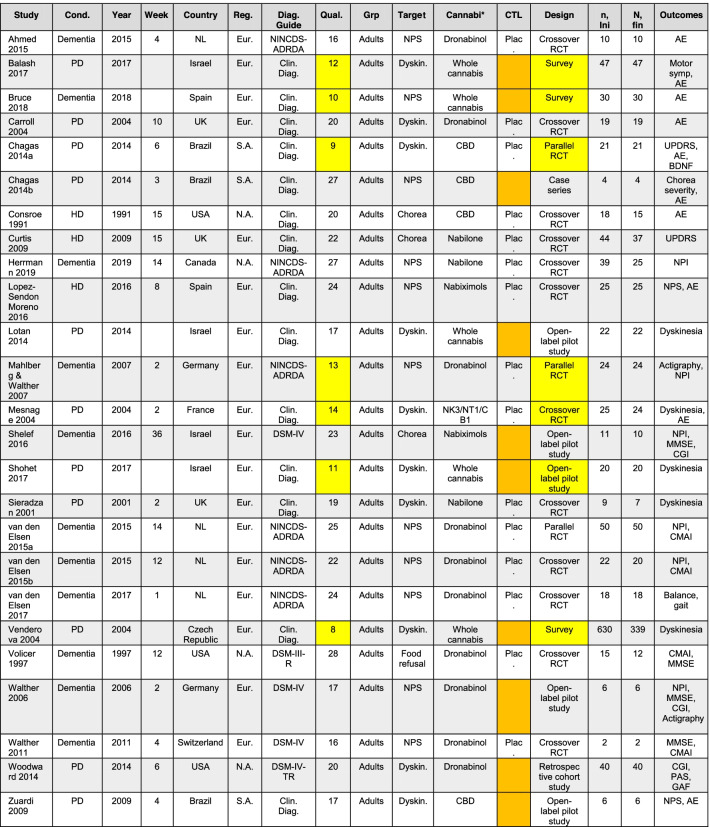
Table 4.
Summary of study parameters
Abbreviations: AD (dementia) Alzheimer’s disease, AE adverse event, BDNF brain-derived neurotrophic factor, CGI Clinical Global Impression, Clin. Diag. clinical diagnosis, Cond. condition (diagnosis), CMAI Cohen-Mansfield Agitation Inventory, Diag. Guide diagnostic guide), DSM Diagnostic and Statistical Manual of Mental Disorders (version III or IV or IV-TR/Text Revision), Dyskin. dyskinesia, Eur. Europe, GAF Global Assessment of Functioning, HD Huntington’s disease, MMSE mini-mental state examination, N.A. North America, NINCDS-ADRDA National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, NL Netherlands, NPI neuropsychiatric inventory, NPS neuropsychiatric symptoms, PD Parkinson’s disease, PAS Pittsburgh Agitation Scale, Plac placebo, Reg. region, RCT randomized controlled trial, S.A. South America, UPDRS Unified PD Rating Scale, UK United Kingdom, USA United States of America, Week week (treatment duration), Year year (of study), Yellow highlights: studies with a ‘poor’ quality rating (< 14 on the Modified Downs and Black Checklist (Appendix 2); Orange highlights: studies lacking a placebo treatment