Abstract
OBJECTIVES
Surgery is the standard treatment in early-stage non-small-cell lung cancer and select cases of small-cell lung cancer, but gender differences in its use and outcome are poorly known. Gender differences in surgical resection rates and long-term survival after lung cancer surgery were therefore investigated.
METHODS
In Finland, 3524 patients underwent resection for primary lung cancer during 2004–2014. Surgical rate and mortality data were retrospectively retrieved from 3 nationwide compulsory registries. Survival was studied by comparing propensity-matched cohorts. Median follow-up was 8.6 years.
RESULTS
Surgery rate was higher in women (15.9% vs 12.3% in men, P < 0.0001). Overall survival was 85.3% 1 year, 51.4% 5 years, 33.4% 10 years and 24.2% at 14 years from surgery. In matched groups, survival after resection was better in women after 1 year (91.3% vs 83.3%), 5 years (60.2% vs 48.6%), 10 years (43.7% vs 27.9%) and 14 years (29.0% vs 21.1%) after surgery [hazard ratio (HR) 0.66; confidence interval (CI) 0.58–0.75; P < 0.0001]. Of all first-year survivors, 39.1% were alive 10 years and 28.3% 14 years after surgery. Among these matched first-year survivors, women had higher 14-year survival (36.9% vs 25.3%; HR 0.75; CI 0.65–0.87; P = 0.0002).
CONCLUSIONS
Surgery is performed for lung cancer more often in women. Women have more favourable short- and long-term outcome after lung cancer surgery. Gender discrepancy in survival continues to increase beyond the first year after surgery.
Keywords: Lung cancer, Cohort study, Gender differences, Long-term survival, Outcomes, Propensity score matching, Surgery rate, Survival
INTRODUCTION
Surgery is the standard of care for localized and locally advanced non-small-cell lung cancer (NSCLC). Overall survival is 66% for stage IA lung cancer, 43% for stage IIA and 23% for stage IIIA lung cancer after 5 years. Median survival is 95 months for stage IA and 19 months for stage IIIA lung cancer [1]. Incidence and mortality for lung cancer have traditionally been higher in men, but the gender gap has been narrowing, irrespective of changing smoking habits, steadily with an increasing incidence of lung cancer in women [2]. In addition to epidemiological variation, sex differences exist in aetiology, comorbidities and presentation of lung cancer. Women are more often non-smokers with a more favourable comorbidity profile, better lung function and lower disease stage [3]. Therefore, it seems reasonable that the resection rate is higher and the surgical morbidity is lower in women [4, 5].
In general, female sex has been proposed to be a good prognostic factor for long-term survival [6]. Survival after surgery was found to be better in females in a population-based study from Norway [7].
Detailed data on gender differences following surgery for lung cancer in a nationwide setting are scarce. The aim of the current study is to assess the prognostic role of gender in survival after lung cancer resection at the population level in Finland. We provide data for significant gender differences in individuals operated on between 2004 and 2014.
PATIENTS AND METHODS
Ethical statement
Our research data are based on mandatory health registers, which are collected on the basis of Finnish health register legislation without informed consent. The use of register data is possible after the data keeping organizations have authorized the use of their data.
Legal basis for processing of personal data is public interest and scientific research [EU General Data Protection Regulation 2016/679 (GDPR), Article 6(1)(e) and Article 9(2)(j); Data Protection Act, Sections 4 and 6].
The study was approved by The National Institute for Health and Welfare of Finland (permissions no: THL/143/5.05.00/2015 and THL/707/5.05.00/2016), Statistics Finland (TK53-484–20) and the Finnish Cancer Registry, individual patient consent was not required based on the retrospective registry design.
Study design and data collection
We studied gender differences in surgery rates and survival after lung or tracheal cancer surgery in population-based nationwide registry cohort follow-up design. All patients aged ≥16 years who underwent lung resection (lobectomy, segmentectomy or extra anatomic resection were identified by NOMESCO codes) between 1 January 2004 and 31 December 2014 were retrospectively identified from the Care Register for Healthcare in Finland registry held by The National Institute for Health and Welfare of Finland [8]. This obligatory, nationwide, registry includes data on all hospital admissions in Finland [9]. Discharge diagnoses (International Classification of Diagnosis/ICD-10) and operational codes (Nordic Classification of Surgical Procedures) were obtained from Care Register for Healthcare in Finland. Data on cancer morphology (ICD-03), stage and adjuvant therapies were obtained from the Finnish Cancer Registry. The obligatory and nationwide Cancer Registry receives notifications of tumours independently from multiple sources at different phases of the disease resulting in high coverage of cancer cases [10]. Mortality data were obtained from obligatory and nationwide cause of death registry held by Statistics Finland. For calculation of the surgical rate, age- and sex-specific data of new onset lung or trachea cancers in Finland during the study period were obtained from the Finnish Cancer Registry [11]. The STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) checklist for cohort studies was observed in the preparation of the manuscript.
All operated patients with a primary malignant neoplasm of the lung or trachea (ICD-10 codes C33-C34) in the cancer registry or cause of death registry were included in surgery rate analyses. Survival after surgery was analysed in all operated patients and in patients who survived the first year after surgery. For survival analyses, propensity score matching was used to identify groups of men and women with comparable baseline characteristics. Follow-up ended 14 years after surgery or 31 December 2018, whichever came first.
Statistical analysis
Effect sizes in baseline characteristics between study-groups were evaluated with standardized difference scores [9]. Charlson comorbidity index including malignancies other than lung or tracheal cancer, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes mellitus, hemi- or paraplegia, renal disease and AIDS/HIV was calculated according to a previously published algorithm [12]. Patient characteristics listed in Table 1 were used in creation of propensity score by using logistic regression. Obtained score was used for local optimal 1:1 calibre matching without replacement using 0.05 calibre width of the logit of the standard deviation. Matching was first performed to all operated patients. In order to re-balance groups of 1-year survivors, re-matching using same variables (Table 1) was performed.
Table 1:
Features of lung cancer resection patients, operations and tumour between sexes. All operated patients and propensity score-matched cohort
|
Variables |
Original cohort |
Matched cohort |
||||
|---|---|---|---|---|---|---|
| Women (n = 1300) | Men (n = 2224) | SD | Women (n = 1223) | Men (n = 1223) | SD | |
| Age (years), mean ± SD | 64.4 ± 10.4 | 66.5 ± 8.9 | 0.21 | 65.1 ± 9.6 | 65.2 ± 9.2 | 0.01 |
| CCI score, n (%) | 0.25 | 0.02 | ||||
| 0–1 | 256 (19.7) | 342 (15.4) | 228 (18.6) | 230 (18.8) | ||
| 2 | 830 (63.9) | 1300 (58.5) | 781 (63.9) | 773 (63.2) | ||
| 3 | 147 (11.3) | 389 (17.5) | 147 (12.0) | 148 (12.1) | ||
| ≥4 | 67 (5.2) | 193 (8.7) | 67 (5.5) | 72 (5.9) | ||
| Operation type, n (%) | 0.25 | 0.02 | ||||
| Pneumonectomy | 81 (6.2) | 273 (12.3) | 80 (6.5) | 78 (6.4) | ||
| Lobectomy/bilobectomy | 1072 (82.5) | 1797 (80.8) | 1030 (84.2) | 1027 (84.0) | ||
| Sublobar resection | 147 (11.3) | 154 (6.9) | 113 (9.2) | 118 (9.7) | ||
| Usage of VATS, n (%) | 326 (25.1) | 384 (17.3) | 0.19 | 286 (23.4) | 293 (24.0) | 0.01 |
| Adjuvant chemotherapy, n (%) | 251 (19.3) | 461 (20.7) | 0.04 | 246 (20.1) | 244 (20.0) | 0.00 |
| Adjuvant radiotherapy, n (%) | 310 (13.9) | 148 (11.4) | 0.08 | 148 (12.1) | 142 (11.6) | 0.02 |
| Morphology, n (%) | 0.54 | 0.06 | ||||
| Adenocarcinoma | 786 (60.5) | 998 (44.9) | 756 (61.8) | 770 (63.0) | ||
| Squamous cell carcinoma | 234 (18.0) | 872 (39.2) | 234 (19.1) | 233 (19.1) | ||
| Atypical carcinoid tumour | 139 (10.7) | 83 (3.7) | 94 (7.7) | 78 (6.4) | ||
| Small cell carcinoma | 30 (2.3) | 52 (2.3) | 30 (2.5) | 35 (2.9) | ||
| Other/carcinoma NAS | 111 (8.5) | 219 (9.9) | 109 (8.9) | 107 (8.8) | ||
| Stage, n (%) | 0.17 | 0.03 | ||||
| Isolated tumour | 520 (37.6) | 887 (39.9) | 499 (40.8) | 496 (40.6) | ||
| Spread only to regional lymph nodes | 184 (14.2) | 359 (16.1) | 176 (14.4) | 168 (13.7) | ||
| Metastasized or invasion to adjacent structures | 100 (7.7) | 262 (11.8) | 99 (8.1) | 96 (7.9) | ||
| Unknown | 496 (38.2) | 716 (32.2) | 449 (36.7) | 463 (37.9) | ||
| Centre size (>16 annual resections), n (%) | 909 (69.9) | 1395 (62.7) | 0.15 | 835 (68.3) | 848 (69.3) | 0.05 |
All baseline variables were included in the propensity matching procedure.
CCI: Charlson comorbidity index; NAS: not otherwise specified; SD: standardized difference; VATS: video-assisted thoracic surgery.
Gender differences in surgical rate were studied with Z-test. Survival was studied using the Kaplan–Meier method. Cox regression models were used for studying mortality hazard with male sex as reference. Analyses of non-matched cohorts were performed using both unadjusted univariate and adjusted multivariate models. Baseline features listed in Table 1 were used in multivariate Cox models. Proportional hazard assumptions were confirmed by visual examination of Schoenfeld residuals. Admission duration from surgery to discharge (full days) was studied using negative binomial regression. Follow-up time was evaluated using reverse Kaplan–Meier method. Results are given as mean, median, percentage or hazard ratio (HR) with 95% confidence interval (CI), ±standard deviation or interquartile range (IQR). P-value <0.05 was considered statistically significant. Analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
There were 3254 first-time resections for lung cancer in Finland during the study period, of whom 36.9% (n = 1300) were women and 63.1% were men (n = 2224). Women were slightly younger than men and had lower burden of comorbidities (Table 1). Men were more commonly treated with pneumonectomy, while women had sublobar resection more commonly. Usage of video-assisted thoracic surgery was more common in women. Tumour histology and stage differed between sexes. Adenocarcinoma and atypical carcinoid tumour were more common in women while squamous cellular carcinoma was notably more common in men. Known metastasis or invasion to adjacent structures was more common in men. Usage of adjuvant therapies was similar in both men and women.
Surgical resection rate
During the study period, 26796 new lung cancers occurred in Finland. The overall surgical resection rate in the original cohort was 13.4%. The overall rate of surgery was 29% higher in women compared to men (15.9% vs 12.3%, P < 0.001). Youngest patients (<50 years) with cancer were most commonly treated with surgery and the difference between genders was highest (52.7% in women and 26.6% in men, P < 0.001) in this patient subpopulation (Fig. 1). A gender difference in treatment approach was present in the older age group with men aged 50–74 years significantly less likely to be treated with surgery (P = 0.01). The gender difference was no longer present in the oldest patients aged ≥75 years (Fig. 1).
Figure 1:
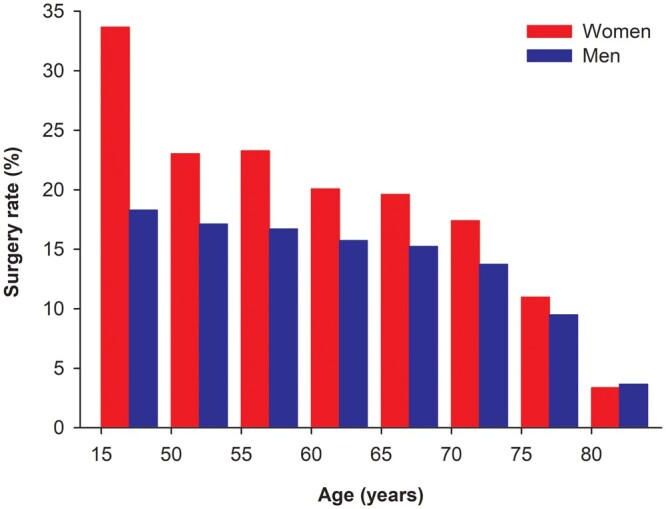
Lung cancer resection rates in Finland 2004–2014 by sex and age.
Survival
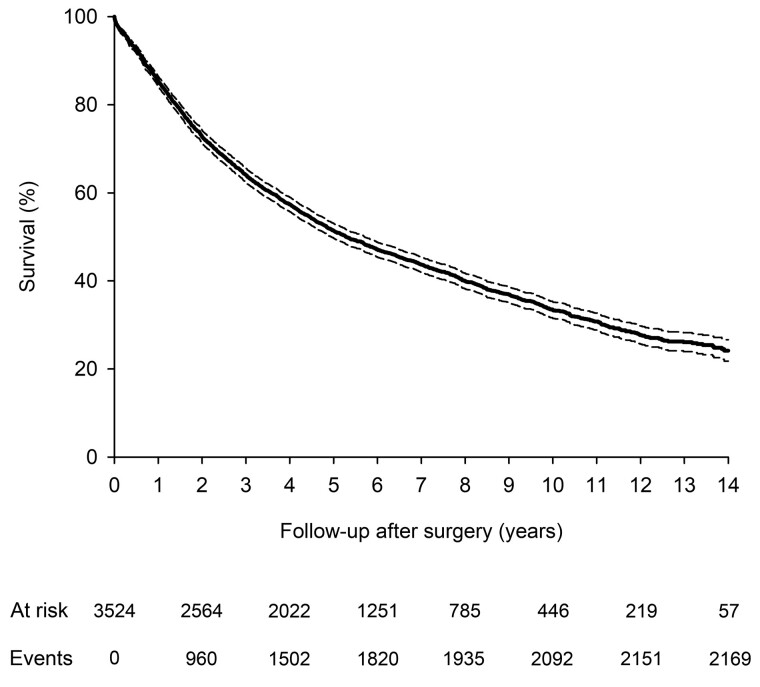
The overall survival rate after lung cancer surgery in the original cohort was 97.8% (CI 97.2–98.2%) at 30 days, 85.3% (CI 84.1–86.4%) at 1 year, 51.4% (CI 49.8–53.1%) at 5 years, 33.4% (31.5–35.2%) at 10 years and 24.2% (21.8–26.6%) at 14 years after lung cancer surgery (Fig. 2). Propensity score matching resulted in groups of 1223 women and 1223 men patients with balanced baseline characteristics (Table 1). Median follow-up was 8.6 (IQR 8.4–8.8) years for original and 8.4 (IQR 5.9–11.3) for matched cohorts with no difference between treatment groups [8.5 (IQR 6.0–11.9) years in women vs 8.4 (IQR 5.9–11.2) in men, P = 0.323, matched cohort]. The corresponding survival rates were 98.1%, 87.3%, 54.4%, 35.8% and 27.4% in the matched cohort. Gender differences in mortality and admission duration were studied in the propensity-matched cohort.
Figure 2:
Cumulative survival of all operated lung cancer patients. Dashed lines represent 95% confidence intervals.
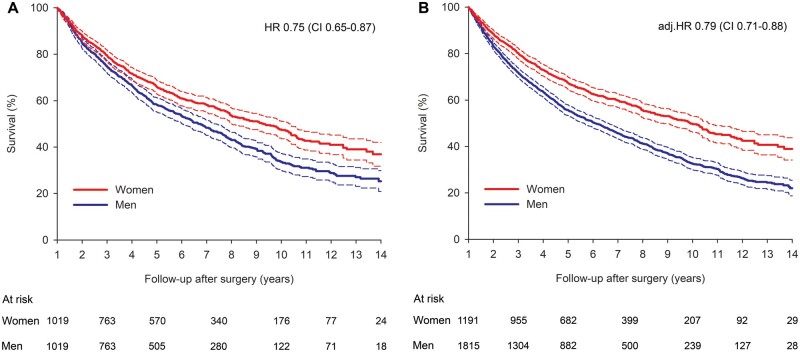
Median duration of admission was 8 days (IQR 4) in men and 7 (IQR 3) in women (P = 0.011). Survival after lung cancer surgery was significantly higher in women (Fig. 3A).
Figure 3:
Cumulative survival after lung cancer resection in propensity-matched patients (A) and all patients (B) by sex. Dashed lines represent 95% confidence intervals. Adj. HR: adjusted HR; CI: confidence interval; HR: hazard ratio.
Ninety-day mortality was 5.3% in men and 1.7% in women (HR 0.31, CI 0.19–0.52; P < 0.001). One-year survival was 91.3% (CI 89.5–92.7%) in women compared to 83.3% in men (CI 81.1–85.3%) with HR for mortality of 0.46 (CI 0.36–0.60; P < 0.001). Five years after the operation, survival was 60.2% (57.4–63.0%) in women and 48.6% (CI 45.7–51.3%) in men (HR 0.67; CI 0.58–0.77, P < 0.001). Ten years after surgery, 43.7% (CI 40.3–47.0%) of operated women and 27.9% (24.8–31.1%) of operated men were alive (HR 0.66, CI 0.58–0.75; P < 0.001). Cumulative survival at 14 years after surgery was 29.0% (21.9–35.6%) in women and 21.1% (CI 17.4–25.0%) in men (HR 0.66, CI 0.58–0.75; P < 0.001). Results were comparable in the original cohort [14-year survival 35.7% (31.3–40.1%) vs 18.0% (CI 15.3–20.8%)] with unadjusted HR of 0.61 (0.55–0.67) and adjusted HR of 0.72 (0.65–0.79; P < 0.001 for both) (Fig. 3).
Of all operated patients, 3006 were alive after the first year following surgery (60.4% women). Among these patients, the 5-year survival rate after surgery was 60.2% (CI 58.5–62.0%), the 10-year survival rate after surgery was 39.1% (CI 37.0–41.2%) and the 14-year survival rate was 28.3% (CI 25.5–31.2%). Re-matching of 1-year survivors resulted in 1019 patient pairs with comparable baseline features. Overall 10-year survival rate after surgery in the re-matched cohort was 40.4%. Poorer long-term outcome after lung cancer resection in men continued also in first-year survivors (Fig. 4). Five-year survival in men was 58.3% (55.2–61.3%) vs 66.0% (62.9–68.8%) in women (HR 0.78, CI 0.67–0.91; P = 0.002). Of first-year female survivors, 47.3% (43.6–51.0%) were alive 10 years after surgery, while 33.5% (29.8–37.2%) of men survived 10 years (HR 0.75, CI 0.65–0.87; P > 0.001). At 14 years after surgery, the survival rate was 36.9% (CI 31.8–42.1%) for women and 25.3% (CI 20.9–29.9%) for men (HR 0.75, CI 0.65–0.87; P > 0.001).
Figure 4:
Outcome of first-year survivals after lung cancer resection surgery in propensity-matched patients (A) and all patients (B) by sex. Dashed lines represent 95% confidence intervals. Adj. HR: adjusted HR; CI: confidence interval; HR: hazard ratio.
Results were comparable in original cohort of 1-year survivors with 14-year survival of 22.0% (CI 18.7–25.4%) in men and 39.0% (34.2–43.7%) in women [unadjusted HR 0.68 (CI 0.61–0.75) and adjusted HR 0.79 (0.71–0.88), P < 0.001 for both].
DISCUSSION
This nationwide registry study found a difference in the resection rate and survival after surgery for primary lung cancer between genders in favour of women. The difference in survival was most pronounced in the early postoperative period, but remained considerable and increased during 5- and 10-year follow-up. Although the present study describes survival differences between genders specifically in the setting of a Finnish healthcare system, the intrinsic mechanisms and possible reasons for the gender-gap are reasonably generalizable to surgically treated lung cancer patients in general. Notably, even as the present study also describes a difference in surgical resection rates between male and female lung cancer patients, it does not sufficiently explain the survival inequality.
Survival differences between genders have been previously reported also by other studies [6, 13, 14]. In these national data from Finland, the findings are similar. Most studies on gender-associated differences in lung cancer survival have found marked differences in demographics between sexes. Women are predominantly younger and are diagnosed in earlier stages and they have a predominance for adenocarcinoma compared to men [14]. Female sex, in a Norwegian population-based study, was, an independent favourable prognostic factor irrespective of age, stage, operation type or histology [7]. In addition to these factors, we were able to adjust for comorbidity burden and adjuvant therapies with the results still favouring females. Whether this sex difference is stage dependent has been debated. In single-centre series, this prognostic benefit has been detected in every stage group (stages I, II and III) or only in early-stage disease [15, 16]. However, in the largest international database, female sex was a prognostic factor only in early-stage cases [1].
In early-stage NSCLC and in select cases of small-cell lung cancer, the treatment of choice is a complete surgical resection [17]. For surgical risk evaluation, important factors are exercise capacity, lung function and comorbidities. Poor exercise capacity, decreased lung functions and comorbidities, in addition to otherwise increased surgical risk, are associated with decreased long-term survival [18–21]. In the current study, the surgical rate was significantly higher in women. Men had, on the other hand, in this stage-adjusted analysis, markedly decreased early survival. Although reasons for these sex discrepancies are unclear, higher 90-day mortality in men strongly suggests men being poorer surgical candidates [22]. Women, in a recent Spanish nationwide prospective cohort study, were less often current or ex-smokers, had better lung functions and fewer comorbidities [3]. Even as our analysis was adjusted for Charlson comorbidity index, the outcome, severity and treatment of various comorbidities included in the index, especially cardiovascular diseases and chronic obstructive pulmonary disease, may vary between sexes [23, 24]. For example, lower FEV1, as seen in the Spanish study in men, is associated with increased cardiovascular and all-cause mortality [3]. These findings imply that women are overall better candidates for surgical treatments which could be related to the long-term survival as well.
Studies have shown that increasing the resection rates for lung cancer improves cancer survival and the overall surgical rate in the present study is considerably lower than suggested, for example, by a study from the UK [25]. While efforts should be made to address gender-specific differences in surgical treatment, a key step in improving cancer outcomes in Finland should be efforts aimed at offering surgery to more patients of both genders. Although there has been a trend in increasing use of surgery for lung cancer [11], the 13.8% rate in the later study period still leaves room for improvement.
These registry-based data could, unfortunately, not quantify the effects of lifestyle and socio-economic factors on long-term survival after lung cancer surgery. Socio-economic inequalities associated with smoking exist in lung cancer mortality in many European countries [26]. In Denmark, the number of smoked cigarettes and high-risk alcohol intake have been associated with decreased early survival in stage I lung cancer [27]. In early-stage disease, continued smoking increases the risk of all-cause mortality and disease recurrence, as well [28]. A striking difference in lung cancer mortality between men and women of low educational level exists in Finland [26] and Finnish males have been shown to consume more both cigarettes and alcohol [29]. As women are more often non-smokers and less affected by low socio-economic status, they less often face the increased premature mortality related to these factors. Before these multiple patient-related and socio-economic factors can be reliably adjusted for, it is difficult to answer the question of what the potential role and differences in tumour biology are between men and women.
Limitations
The first strength of this study is the large nationwide population-based design. Nationwide inclusion of all surgical lung cancer patients with good matching of cofounders enabled us to evaluate the survival differences between sexes reliably. The second strength is the complete follow-up data. The obligatory and nationwide registry data with ability to link cancer patients between registries due to permanent identity numbers of all Finnish citizens enabled follow-up without losses. There are, however, several limitations in the present study. A potential bias could be the inclusion of small-cell lung cancer cases; these, however, were included because the number from the registries used to calculate for surgical resection rates i.e. the number of newly diagnosed lung cancer cases nationwide includes all histologic subtypes of primary lung cancer. The number of operated small-cell lung cancer cases, however, was under 3% (Table 1) and analyses with these cases excluded did not alter the results nor the conclusions (data not shown). A weakness is the lack of standard tumour, node and metastasis (TNM) stage classification. The staging used by the Finnish Cancer Registry is less granular compared to the detailed TNM classification, but is not likely to influence sexes differently, as it still differentiates between local and advanced stages. Furthermore, the present registry has the inherent limitations in evaluating the role of underlying causes for sex-related difference in mortality such as smoking, severity of comorbidities and tumour biology. Specific subgroup survival analyses or, for example, progression-free survival differences cannot be performed from these registry-based data and would require reviewing individual patient records.
CONCLUSIONS
In conclusion, our study showed that female gender was associated with a more favourable short- and long-term outcomes after surgery for lung cancer. In addition, women also have a higher resection rate than men. These differences in access to surgical treatment should warrant a re-evaluation of the treatment pathways in lung cancer to mitigate sex differences. Further studies and in clinical practice vigilant follow-up after surgery are suggested also to identify non-cancer-related co-morbidities as well as socio-economic factors as possible intervention targets in order to further improve the overall survival in these patients.
FUNDING
This work was supported by the Finnish governmental VTR-funding and Finnish Cultural Foundation.
Conflict of interest: Professor Jarmo Gunn declares an unrestricted research grant unrelated to the study subject from Vifor Pharma Ltd.
Author contributions
Anna Lautamäki: Methodology; Validation; Writing—original draft; Writing—review & editing. Jarmo Gunn: Methodology; Project administration; Supervision; Validation; Writing—original draft; Writing—review & editing. Jussi Sipilä: Conceptualization; Methodology; Writing—review & editing. Päivi Rautava: Funding acquisition; Methodology; Project administration; Writing—review & editing. Eero Sihvo: Methodology; Supervision; Writing—review & editing. Ville Kytö: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Hasan Fevzi Batirel, Nuria M. Novoa and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- CI
Confidence interval
- HR
Hazard ratio
- IQR
Interquartile range
- NSCLC
Non-small-cell lung cancer
- TNM
Tumour, node and metastasis
REFERENCES
- 1. Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P.. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009;4:792–801. [DOI] [PubMed] [Google Scholar]
- 2. Locher C, Debieuvre D, Coëtmeur D, Goupil F, Molinier O, Collon T. et al. Major changes in lung cancer over the last ten years in France: the KBP-CPHG studies. Lung Cancer 2013;81:32–8. [DOI] [PubMed] [Google Scholar]
- 3. Fibla JJ, Molins L, Quero F, Izquierdo JM, Sánchez D, Hernández J. et al. ; on behalf of the Group of Postoperative Complications of the Spanish Society of Thoracic Surgeons (GCP-SECT). Perioperative outcome of lung cancer surgery in women: results from a Spanish nationwide prospective cohort study. J Thorac Dis 2019;11:1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belot A, Fowler H, Njagi EN, Luque-Fernandez MA, Maringe C, Magadi W. et al. Association between age, deprivation and specific comorbid conditions and the receipt of major surgery in patients with non-small cell lung cancer in England: a population-based study. Thorax 2019;74:51–9. [DOI] [PubMed] [Google Scholar]
- 5. Infante MV, Benato C, Silva R, Rocco G, Bertani A, Bertolaccini L. et al. What counts more: the patient, the surgical technique, or the hospital? A multivariable analysis of factors affecting perioperative complications of pulmonary lobectomy by video-assisted thoracoscopic surgery from a large nationwide registry. Eur J Cardiothorac Surg 2019;56:1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chatkin JM, Abreu CM, Fritscher CC, Wagner MB, Pinto JA.. Is there a gender difference in non-small cell lung cancer survival? Gend Med 2004;1:41–7. [DOI] [PubMed] [Google Scholar]
- 7. Strand TE, Bartnes K, Rostad H.. National trends in lung cancer surgery. Eur J Cardiothorac Surg 2012;42:355–8. [DOI] [PubMed] [Google Scholar]
- 8. Valo JK, Kyto V, Sipila J, Rautava P, Sihvo E, Gunn J.. Thoracoscopic surgery for lung cancer is associated with improved survival and shortened admission length: a nationwide propensity-matched study. Eur J Cardiothorac Surg 2020;57:100–6. [DOI] [PubMed] [Google Scholar]
- 9. Kyto V, Myllykangas ME, Sipila J, Niiranen TJ, Rautava P, Gunn J.. Long-term outcomes of mechanical vs biologic aortic valve prosthesis in patients older than 70 years. Ann Thorac Surg 2019;108:1354–60. [DOI] [PubMed] [Google Scholar]
- 10. Leinonen MK, Miettinen J, Heikkinen S, Pitkaniemi J, Malila N.. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer 2017;77:31–9. [DOI] [PubMed] [Google Scholar]
- 11. Gunn J, Valo J, Sipila J, Rautava P, Sihvo E, Kyto V.. Trends and results of lung cancer surgery in Finland between 2004 and 2014. Eur J Cardiothorac Surg 2018;54:127–33. [DOI] [PubMed] [Google Scholar]
- 12. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 13. Visbal AL, Williams BA, Nichols FC, Marks RS, Jett JR, Aubry M-C 3rd. et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg 2004;78:209–15; discussion 215. [DOI] [PubMed] [Google Scholar]
- 14. Ferguson MK, Wang J, Hoffman PC, Haraf DJ, Olak J, Masters GA. et al. Sex-associated differences in survival of patients undergoing resection for lung cancer. Ann Thorac Surg 2000;69:245–9; discussion 249–50. [DOI] [PubMed] [Google Scholar]
- 15. Scaglia NC, Chatkin JM, Pinto JA, Tsukazan MT, Wagner MB, Saldanha AF.. Role of gender in the survival of surgical patients with nonsmall cell lung cancer. Ann Thorac Med 2013;8:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerfolio RJ, Bryant AS, Scott E, Sharma M, Robert F, Spencer SA. et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest 2006;130:1796–802. [DOI] [PubMed] [Google Scholar]
- 17. Flehinger BJ, Kimmel M, Melamed MR.. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest 1992;101:1013–8. [DOI] [PubMed] [Google Scholar]
- 18. Sato S, Nakamura M, Shimizu Y, Goto T, Koike T, Ishikawa H. et al. The impact of emphysema on surgical outcomes of early-stage lung cancer: a retrospective study. BMC Pulm Med 2019;19:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shewale JB, Correa AM, Brown EL, Leon-Novelo LG, Nyitray AG, Antonoff MB. et al. Time trends of perioperative outcomes in early-stage non-small cell lung cancer resection patients. Ann Thorac Surg 2020;109:404–11. [DOI] [PubMed] [Google Scholar]
- 20. Iizasa T, Suzuki M, Yasufuku K, Iyoda A, Otsuji M, Yoshida S. et al. Preoperative pulmonary function as a prognostic factor for stage I non-small cell lung carcinoma. Ann Thorac Surg 2004;77:1896–902; discussion 1902–3. [DOI] [PubMed] [Google Scholar]
- 21. Hamada K, Irie M, Fujino Y, Hyodo M, Hanagiri T.. Prognostic value of preoperative exercise capacity in patients undergoing thoracoscopic lobectomy for non-small cell lung cancer. Lung Cancer 2019;128:47–52. [DOI] [PubMed] [Google Scholar]
- 22. Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, Lambe M.. Sex and survival in non-small cell lung cancer: a nationwide cohort study. PLoS One 2019;14:e0219206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alfredsson J, Green JB, Stevens SR, Reed SD, Armstrong PW, Angelyn Bethel M. et al. ; on behalf of the TECOS Study Group. Sex differences in management and outcomes of patients with type 2 diabetes and cardiovascular disease: a report from TECOS. Diabetes Obes Metab 2018;20:2379–88. [DOI] [PubMed] [Google Scholar]
- 24. Åberg J, Hasselgren M, Montgomery S, Lisspers K, Ställberg B, Janson C. et al. Sex-related differences in management of Swedish patients with a clinical diagnosis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2019;14:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riaz SP, Lüchtenborg M, Jack RH, Coupland VH, Linklater KM, Peake MD. et al. Variation in surgical resection for lung cancer in relation to survival: population-based study in England 2004-2006. Eur J Cancer 2012;48:54–60. [DOI] [PubMed] [Google Scholar]
- 26. Van der Heyden JH, Schaap MM, Kunst AE, Esnaola S, Borrell C, Cox B. et al. Socioeconomic inequalities in lung cancer mortality in 16 European populations. Lung Cancer 2009;63:322–30. [DOI] [PubMed] [Google Scholar]
- 27. Christensen NL, Lokke A, Dalton SO, Christensen J, Rasmussen TR.. Smoking, alcohol, and nutritional status in relation to one-year mortality in Danish stage I lung cancer patients. Lung Cancer 2018;124:40–4. [DOI] [PubMed] [Google Scholar]
- 28. Parsons A, Daley A, Begh R, Aveyard P.. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peters SA, Huxley RR, Woodward M.. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open 2014;4:e005663. [DOI] [PMC free article] [PubMed] [Google Scholar]