Abstract
Background
Preterm birth occurs in up to 6% to 10% of all births and is the major complication of pregnancy associated with perinatal mortality and morbidity. Previous preterm delivery is a strong predictor for preterm labour, and the earlier the birth, the more likely it is to be repeated at the same gestation. In the acute setting, betamimetics can decrease contraction frequency or delay preterm birth by 24 to 48 hours.
Objectives
To assess the effectiveness of prophylactic oral betamimetics for the prevention of preterm labour and birth for women with singleton pregnancies at high risk of preterm delivery.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (October 2010) and reference lists.
Selection criteria
Randomised controlled trials in singleton pregnancies at high risk of preterm labour comparing prophylactic oral betamimetics with placebo or any intervention with the specific aim of preventing preterm birth.
Data collection and analysis
Two authors independently assessed trial quality and extracted data.
Main results
One trial (64 singleton pregnancies) was included. The trial compared the oral betamimetic agent isoxuprine with placebo. No difference was seen for perinatal mortality rate (risk ratio (RR) 4.74, 95% confidence interval (CI) 0.50 to 45.00). There was no evidence of an effect of oral betamimetic agents in reduction of spontaneous onset of preterm labour (RR 1.07, 95% CI 0.14 to 8.09) or preterm birth, less than 37 weeks' gestation. There was no significant association between the use of oral betamimetics and side effects sufficient to stop therapy (RR 2.51, 95% CI 0.59 to 10.76). No differences were found for infant outcomes; birthweight less than 2500 grams (RR 1.74, 95% CI 0.44 to 6.87) or neonatal death (RR 4.74, 95% CI 0.50 to 45.00). This trial had adequate methodological quality; however the sample size was inappropriate to determine any significance in neonatal outcome differences between the treatment groups.
Authors' conclusions
There is insufficient evidence to support or refute the use of prophylactic oral betamimetics for preventing preterm birth in women at high risk of preterm labour with a singleton pregnancy.
Plain language summary
Prophylactic oral betamimetics for preventing preterm labour in singleton pregnancies
There are insufficient data on use of betamimetic drugs given by mouth to reduce preterm birth in women at increased risk of preterm labour and carrying one baby.
Women sometimes go into labour early and babies are born prematurely (before 37 weeks). These babies are at increased risk of health problems and the earlier a baby is born the higher the risk. Babies born before 32 weeks have considerable problems, with those born before 28 weeks being at most risk. These babies can suffer from problems with breathing, bleeding, gut and intestines. They are also at increased risk of cerebral palsy or long‐term handicap, and some babies do not survive even the early weeks. Babies need special care, sometimes intensive care, and this can be quite traumatic for parents. Preterm birth occurs in around 6% to 10% of births. Many interventions have been assessed to try to improve outcomes for these babies; this review looks at a group of drugs called betamimetics given by mouth in women at increased risk and carrying one baby. These drugs aim to reduce and inhibit labour contractions. However, they do have side effects which include nausea, vomiting, tremor, headaches and shortness of breath. The review of studies found only one trial involving 64 women. There is therefore insufficient evidence to support the use of these drugs to reduce preterm labour and birth.
Background
Preterm birth occurs in up to 6% to 10% of all births and is the major complication of pregnancy associated with perinatal mortality and morbidity (Lumley 2003). Nearly half of all preterm births are due to preterm labour. Once congenital anomalies are excluded, preterm birth is responsible for the majority of neonatal deaths and is the major cause of disability in childhood (Hack 1999). Most mortality and morbidity occur with extreme prematurity prior to 32 weeks and especially before 28 weeks. Preterm infants often require intensive care. This is associated with emotional and economic costs to both the family and society (Petrou 2001). However, whilst the incidence of death and long‐term handicap is relatively low in those born after 32 weeks, they make up over 80% of all preterm deliveries and, therefore, this group of infants has a significant impact upon the burden that preterm birth makes on public health (Kramer 2000). Preterm birth is associated with respiratory distress syndrome (a respiratory disorder that is characterised by failure of the immature lungs to expand and contract properly during breathing), intracranial haemorrhage (bleeding within the brain), necrotising enterocolitis (a serious gastrointestinal disease in neonates characterised by necrosis of part of the intestine), bronchopulmonary dysplasia (a chronic lung condition that is caused by tissue damage to the lungs and usually occurs in immature infants who have received mechanical ventilation and supplemental oxygen) and cerebral palsy (a disability resulting from damage to the brain before, during, or shortly after birth and outwardly manifested by muscular incoordination and speech disturbances).
Despite intensive research over the past few decades, no decrease in the incidence of preterm labour has occurred, although there have been improved survival and other outcomes for preterm infants. Amongst the risk factors for preterm labour, previous preterm delivery is a strong predictor and the earlier the birth, the more likely it is to be repeated at the same gestation (Hoffman 1984). Overall survival for infants born between 20 and 25 weeks' gestation is in the region of 40% and of those who survive to 30 months morbidity is common, e.g. cerebral palsy in 19% (Costeloe 2000; Wood 2005). It is therefore important to look at measures aimed at preventing recurrence of preterm birth in subsequent pregnancies in women who have experienced a preterm delivery in the past. However, interventions to prevent preterm labour in high‐risk pregnancies have been disappointing. Bed rest is the oldest proposed method for the prevention of preterm birth. A meta‐analysis found not enough evidence to support a policy of routine hospitalisation for bed rest in singleton pregnancies (Sosa 2004). Recent studies demonstrate the benefit of progesterone in reduction of preterm birth in a high‐risk population for preterm birth (da Fonseca 2003; Meis 2003). Betamimetics (isoxsuprine, hexoprenaline, orciprenaline, ritodrine, terbutaline and salbutamol) have been used extensively by obstetricians in the past 20 years in an attempt to pharmacologically inhibit preterm uterine contractions and, thereby, reduce the incidence of preterm delivery, in both acute settings and in the form of prophylaxis (Barden 1980). Beta‐adrenergic agonists activate an enzyme which increases the levels of cyclic adenosine 3',5' monophosphate (cAMP). The increased cellular levels of cAMP decrease myosin light‐chain kinase activity, both by phosphorylation of the myosin light‐chain kinase itself, and by reducing intracellular calcium through increasing calcium uptake by sarcoplasmic reticulum (Gabor 1982).
Betamimetics can decrease contraction frequency or delay preterm birth by 24 to 48 hours when used in the setting of acute preterm labour (Anotayanonth 2004; Gyetvai 1999). As an extrapolation from their effect in the acute setting, oral betamimetics have been used prophylactically in an attempt to prevent preterm labour in twin pregnancy (Yamasmit 2005). The ideal tocolytic agent is one which is effective in prolonging pregnancy but has no side effects for the woman or fetus. As a result of their effect on beta‐adrenergic receptors, betamimetics can cause palpitations, tremor, nausea, vomiting, headaches, nervousness, anxiety, chest pain, shortness of breath and a range of biochemical disturbances such as hyperglycaemia and hypokalaemia. Moreover, pulmonary oedema may occur in about 3% of women, although this is more commonly seen when betamimetics are given intravenously (Sciscione 2003), and has been associated with maternal death. Side effects may necessitate some women stopping the medication. Betamimetics cross the placenta and may cause fetal tachycardia, hypoglycaemia and hyperinsulinism following delivery. Moreover, they can similarly alter in utero fetal heart rate, which may affect fetal/neonatal outcome. It is known that antenatal cardiac events can influence a number of developmental outcomes for infants, particularly neurodevelopmental outcomes such as cerebral palsy and intracranial haemorrhage (Bracci 2006).
Objectives
To assess the effectiveness of prophylactic oral betamimetics for the prevention of preterm labour and birth for women with singleton pregnancies at high risk of preterm delivery.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. We did not include quasi‐randomised controlled trials.
Types of participants
Pregnant women at high risk of preterm delivery with a singleton pregnancy who did not show signs of preterm labour at the time of initiation of therapy.
Types of interventions
Oral betamimetic drugs (any dosage regimen, any agent) compared with placebo or any other intervention (including conservative management) aimed at decreasing preterm labour and preterm birth.
Types of outcome measures
Primary outcomes
Perinatal mortality. Death at childhood follow up at greater than, or equal to, 12 months of age (corrected for preterm birth) or severe neurodevelopmental disability defined as any one or combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70 or more than two standard deviations below the mean), severe auditory impairment (sensorineural deafness requiring hearing aids) or visual impairment (legal blindness).
Secondary outcomes
Maternal
Preterm labour. Threatened preterm labour ‐ regular uterine contractions resulting in cervical change as determined by vaginal examination or serial transvaginal ultrasound scanning. Prelabour spontaneous rupture of membranes. Side effects sufficient to stop therapy:
mild side effects (e.g. tachycardia; tachypnoea; hypotension; nausea; vomiting; hyperglycaemia; hypokalaemia);
severe side effects (e.g. pulmonary oedema; myocardial ischaemia).
Pregnancy prolongation (interval between randomisation and delivery). Woman not satisfied (including maternal anxiety e.g. Hospital Anxiety and Depression Score and postnatal depression e.g. Edinburgh Score). Number of antenatal hospital admissions. Therapeutic use of tocolytics.
Infant
Birth before 28 completed weeks. Birth before 34 completed weeks. Birth before 37 completed weeks. Gestation at birth. Birthweight less than the third centile for gestational age. Birthweight less than 2500 grams. Respiratory distress syndrome. Use of mechanical ventilation. Duration of mechanical ventilation. Intracranial haemorrhage; all grades (I‐IV), and severe haemorrhage ‐ grade III (ventricles distended with blood) or IV (parenchymal involvement) (Papile 1978). Cystic periventricular leucomalacia defined as cysts detected in the periventricular area on ultrasound, computerised tomography or magnetic resonance imaging. Retinopathy of prematurity; all stages, and of stage three or more based on international classification (ICROP 1984). Chronic lung disease defined as requirement for supplemental oxygen requirement at 36 weeks' postmenstrual age. Necrotising enterocolitis defined using Bell's criteria (or modifications), that is, the presence of at least two of the following features: pneumatosis coli on abdominal X ray; abdominal distension or abdominal radiograph with gaseous distension or frothy appearance of bowel lumen (or both); blood in stool; lethargy, hypotonia or apnea, or combination of these (Bell 1978). Neonatal sepsis. Fetal death or major morbidity (for example, organ failure, prolonged intensive care unit admission, etc). Neonatal death. Admission to neonatal intensive care unit. Neonatal length of hospital stay. Teratogenic effects.
For mother and child
Side effects and adverse effects of betamimetics sufficient to stop medication. Side effects and adverse effects of betamimetics not sufficient to stop medication. Use of health service resources for the woman. Antenatal hospital admission; visits to day care units. Use of intensive care. Use of health service resources for the infant. Admission to special care/intensive care nursery. Use of mechanical ventilation; length of stay in hospital. Developmental and special needs after discharge.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (October 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
For details of searching carried out in the initial version of the review, see:Appendix 1.
Searching other resources
We reviewed cited references from retrieved articles, abstracts, letters to the editor and editorials for additional studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
The two authors screened the studies that were found as a result of the search strategy described earlier. We selected all potential trials for eligibility according to the criteria specified in the protocol using a standardised form. We excluded trials that did not follow the criteria.
The two authors independently assessed trials under consideration for methodological quality. We resolved any disagreements by discussion. We used standard criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005) to assess the trials.
Data extraction and management
The authors extracted data using a data collection form. Where possible, we extracted data to allow an intention‐to‐treat analysis. We sought missing data from investigators of individual trials as necessary. We extracted the following data from each included study: (1) incidence of preterm labour and birth; (2) neonatal and infant outcomes; (3) adverse maternal effects; (4) whether steroids given routinely to all women; and, where recorded, the number and percentage of women given steroids in the intervention and control group. We aimed to explore steroid administration as a possible source of heterogeneity in subgroup analyses. We used the Review Manager software (RevMan 2003) to enter all the data.
Assessment of risk of bias in included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence are described for each trial.
(1) Selection bias (randomisation and allocation concealment)
We assigned each trial a quality score, using the following criteria: (A) adequate concealment of allocation: such as telephone randomisation, consecutively‐numbered, sealed opaque envelopes; (B) unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach; (C) inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week. We would include any trials given this score in initial analysis, and evaluate through sensitivity analyses.
(2) Attrition bias (loss of participants, for example, withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria: (A) less than 5% loss of participants; (B) 5% to 9.9% loss of participants; (C) 10% to 19.9% loss of participants; (D) more than 20% loss of participants.
We excluded trials with more than 20% loss to follow up for outcome measure.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria: (1) blinding of participants (yes/no/unclear); (2) blinding of caregiver (yes/no/unclear); (3) blinding of outcome assessment (yes/no/unclear).
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2003). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, we explored this by sensitivity analysis, followed by a random‐effects analysis if required.
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals. For continuous data, we have used the mean difference if outcomes are measured in the same way between trials. The standardised mean difference has been used to combine trials that measure the same outcome, but use different methods. We have reported evidence of skewness where present.
We have analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Planned subgroup analyses included: (1) type of betamimetic agent; (2) dosage of betamimetic agent administered for different individual betamimetic agents; (3) duration of therapy; (4) steroid administration; (5) setting (income‐rich countries and income‐poor/medium countries as per World Health Organization categorisation: http://www.worldbank.org/data/databytopic/class.htm).
Results
Description of studies
We identified eight studies as potentially eligible for inclusion in this review. We excluded seven trials, so this review includes one trial.
Excluded studies
SeeCharacteristics of excluded studies table.
Included studies
A total of 103 pregnant women participated in the included study comparing the oral betamimetic agent isoxuprine with placebo (Mathews 1967). Only the subset of trial participants who had a singleton pregnancy (64 of 103 participants) was included.
Participants
The trial was conducted in the United Kingdom (UK) (Mathews 1967). Mean age of participants was not described. Mean gestational age at trial entry was 30.6 weeks (range 28 to 32 weeks) (Mathews 1967). It was unclear whether cases with medical or obstetric complications were excluded. Women were described as having 'a higher than average chance of going into premature labour' on the basis of a history of at least one previous spontaneous premature labour occurring between 24 and 36 weeks, with delivery of a baby weighing less than five pounds eight ounces. Further details of previous premature deliveries were not given.
Interventions
The trial compared the oral betamimetic agent isoxuprine with placebo (Mathews 1967) (seeCharacteristics of included studies). The trial stopped the medication at 36 weeks' gestation or, if labour started, medication was stopped when delivery seemed inevitable. Mean length of treatment was not reported and there was no reference to whether steroids were used for fetal lung maturity enhancement. Subgroup analysis of steroid administration was therefore not possible.
Outcomes
The study did not report the incidence of threatened preterm labour. Delivery before 36 weeks' gestation was used as a cutoff for premature delivery (Mathews 1967). However, no description was given of the method used for determining gestational age.
Risk of bias in included studies
The trial was described as a double‐blind study, but the method of randomisation and allocation concealment was not reported (Mathews 1967). No description was given of sample size calculation, although the authors reported that the aim was to recruit 200 women (a combination of singleton and multiple pregnancies) to the trial. The trial was halted at an early stage due to the high incidence of disturbing side effects and the difficulty experienced by patients in taking 12 tablets of betamimetic/placebo in combination with folic acid and iron. Follow up was complete (Mathews 1967).
Effects of interventions
This review includes data from one trial with a total of 64 singleton pregnancies (Mathews 1967). Thirty‐one women were randomised to prescription of the oral betamimetic agent isoxuprine at a dose of 30 mg four times daily, and 33 women were randomised to placebo.
Primary outcomes
No difference was seen for perinatal mortality rate (risk ratio (RR) 4.74, 95% confidence interval (CI) 0.50 to 45.00). The trial did not report results for death at childhood follow up or severe neurodevelopmental disability.
Secondary outcomes
Maternal
There was no evidence of an effect of oral betamimetic agents in reduction of spontaneous onset of preterm labour (RR 1.07, 95% CI 0.14 to 8.09) (Mathews 1967). The trial did not report on threatened preterm labour or prelabour spontaneous rupture of the membranes. There was no significant association between the use of oral betamimetics and side effects/adverse effects, either not sufficient to stop therapy (RR 2.50, 95% CI 0.88 to 7.08) or sufficient to stop therapy (RR 2.51, 95% CI 0.59 to 10.76). However, it was stated that the trial was halted at an early stage due to 'the high incidence of disturbing side effects'. Side effects were categorised as palpitations, cramps or 'other'. No results were reported for patient satisfaction, number of hospital admissions or the use of rescue or additional tocolysis.
Infant
No differences were found for preterm birth (less than 37 weeks) (RR 1.07, 95% CI 0.14 to 8.09) or for other infant outcomes:
birthweight less than 2500 grams (RR 1.74, 95% CI 0.44 to 6.87; 64 infants);
neonatal death (RR 4.74, 95% CI 0.50 to 45.00; 64 infants).
The trial did not formally report on complications of prematurity such as respiratory distress syndrome, intracranial haemorrhage, retinopathy of prematurity or necrotising enterocolitis.
Use of health service resources
The trial did not report on use of antenatal day care units; antenatal hospital admissions; admission to, or length of stay in, the neonatal intensive care unit.
Discussion
The objective of this review was to assess the effectiveness of prophylactic oral betamimetics for the prevention of preterm labour and birth for women with singleton pregnancies at high risk of preterm delivery. However, the results of the single randomised controlled trial that met the eligibility criteria (Mathews 1967) should be interpreted with caution. We considered the trial included in this review to be of reasonable quality, although the allocation concealment was not clearly defined.
The single trial included in this review did not demonstrate a statistically significant difference for the primary outcome of perinatal mortality rate (risk ratio (RR) 4.74, 95% confidence interval (CI) 0.50 to 45.0). However, the sample size was small and hence had limited power to detect small, but important differences, especially in the primary outcomes of perinatal mortality and death at childhood follow up. Indeed, in most estimates of effect size, the 95% CIs are very wide. This limitation was acknowledged by the trial authors. In the 41 years since the this trial was conducted, considerable advances have occurred in neonatal medicine, with significant improvements in the survival of neonates who deliver prematurely. Indeed, this trial (Mathews 1967) predates the first randomised controlled trial in humans of corticosteroids for the prevention of RDS which was conducted in 1972 (Liggins 1972). It is perhaps therefore not surprising that important clinical outcomes, such as the most common complications of prematurity and birth before 28 or 34 completed weeks, were not reported by the trial authors (Mathews 1967).
It is difficult to evaluate the efficacy (and safety) of prophylactic oral betamimetics for the prevention of preterm labour and birth for women with singleton pregnancies at high risk of preterm delivery in the absence of adequately powered RCTs. There is a considerable body of data regarding maternal adverse events and the use of betamimetics in the setting of acute preterm labour. In this setting, betamimetics are significantly associated with: withdrawal from treatment due to adverse effects; chest pain; dyspnea; tachycardia; palpitation; tremor; headaches; hypokalemia; hyperglycemia and nausea or vomiting (Anotayanonth 2004). More limited evidence regarding the use of prophylactic oral betamimetics is available from two Cochrane reviews, one of prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy (Yamasmit 2005) and the other of oral betamimetics for maintenance therapy after threatened preterm labour (Dodd 2006). The review which looked at twin pregnancy included five trials (344 twin pregnancies), all comparing oral betamimetics to placebo. This review reported on maternal death (RR 2.84, 95% CI 0.12 to 68.57; one study, 144 patients), but did not comment upon side effects of treatment. The review which looked at maintenance therapy after threatened preterm labour (11 RCTs) found that, whilst oral betamimetics were not associated with a statistically significant increase in the incidence of side effects sufficient to stop therapy (RR 2.71, 95% CI 0.11 to 64.79), their use was associated with a statistically significant increase in the incidence of tachycardia (RR 1.55, 95% CI 1.02 to 2.37).
It is not possible to discuss health service resource implications of the effects of oral betamimetics on the prevention of preterm labour and birth for women with singleton pregnancies at high risk of preterm delivery, as the study (Mathews 1967) did not include any data on maternal or neonatal hospital admissions.
Authors' conclusions
Implications for practice.
There is insufficient evidence either supporting or refuting the use of prophylactic oral betamimetics for preventing preterm birth in women at high risk of preterm delivery with a singleton pregnancy. The use of oral betamimetics for the prevention of preterm labour and birth in women with singleton pregnancies at high risk of preterm delivery is more or less an unevaluated intervention.
Implications for research.
There is still no effective intervention to prevent preterm birth in women with a singleton pregnancy at high risk of preterm delivery. There are no recent data suggesting that prophylactic oral betamimetics are currently being employed in women with a singleton pregnancy at high risk of preterm delivery. In the developed world women with a pregnancy considered at high risk of preterm delivery are often managed in specialised clinics with non‐randomised cohort data suggesting improved perinatal outcomes with the provision of intensive antenatal education, continuity of carer and individualised care (Bienstock 2001). Such specialised clinics may also see women with previous preterm prelabour rupture of the membranes, previous late miscarriage (16 weeks 0 days to 23 weeks 6 days of gestation) or prior cervical surgery. Prediction of risk may utilise ultrasound assessment of cervical length and the presence of fetal fibronectin in cervico‐vaginal secretions. The package of care offered in such clinics may include promising prophylactic interventions for labour prevention including progesterone, clindamycin or cervical cerclage (Althuisius 1998; da Fonseca 2003; Ugwumadu 2003). Whilst the review authors do not feel that further large RCTs of prophylactic betamimetics are a research priority if future trials are proposed, they should be adequately powered to look at the effect of oral betamimetics on neonatal mortality and the rate of preterm birth. Outcome measurements should include not only the incidence of preterm birth, but also the incidence of precisely defined immaturity‐related neonatal morbidities and longer‐term childhood outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2010 | New search has been performed | Search updated. No new trials identified. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Contact details updated. |
| 4 February 2008 | Amended | Converted to new review format. |
| 19 October 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to Sonja Henderson (Review Group Co‐ordinator), Denise Atherton (Administrative Assistant) and Lynn Hampson (Trials Search Co‐ordinator), Cochrane Pregnancy and Childbirth Group, for their advice and support through the protocol and review process.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search strategies
For the initial version of the review authors searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2006, Issue 3) using the following search strategy:
#1 MeSH descriptor Obstetric Labor, Premature explode all trees #2 preterm* or prematur* #3 ritodrin* or salbutamol or fenoterol or hexoprenalin* or isoxuprin* or isoxsuprin* or terbutalin* or orciprenalin* or nylidrin* #4 beta‐mimetic* or betamimetic* or beta‐agonist* or betaagonist* or beta 2‐mimetic* or beta‐adrenomimetic* or beta‐sympathomimetic* or betasympathomimetic* #5 prevent* #6 (#1 OR #2) #7 (#3 OR #4) #8 (#5 AND #6 AND #7)
We also searched EMBASE (January 1985 to December 2006) using the following search strategy:
Beta‐Adrenergic‐Receptor‐Stimulating‐Agent (explode descriptor)
Premature‐Labor (prevention and control) (descriptor)
1 AND 2
Data and analyses
Comparison 1. Betamimetic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| 2 Preterm labour | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| 3 Side effects sufficient to stop therapy | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.59, 10.76] |
| 4 Birth before 37 completed weeks | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.14, 8.09] |
| 5 Birthweight less than 2500 grams | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.44, 6.87] |
| 6 Neonatal death | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.50, 45.00] |
| 7 Side effects and adverse effects of betamimetics not sufficient to stop medication | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.88, 7.08] |
1.1. Analysis.
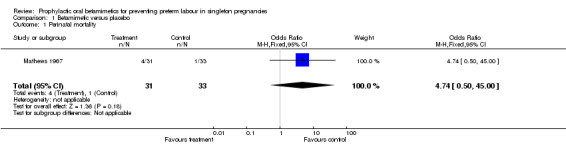
Comparison 1 Betamimetic versus placebo, Outcome 1 Perinatal mortality.
1.2. Analysis.
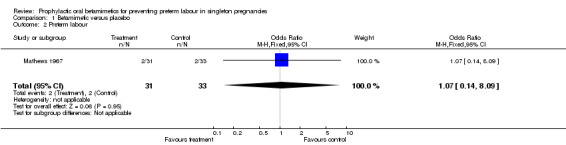
Comparison 1 Betamimetic versus placebo, Outcome 2 Preterm labour.
1.3. Analysis.
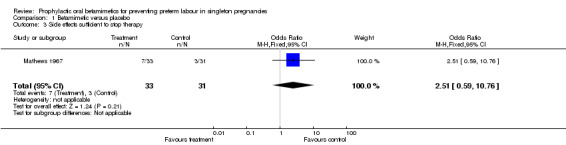
Comparison 1 Betamimetic versus placebo, Outcome 3 Side effects sufficient to stop therapy.
1.4. Analysis.
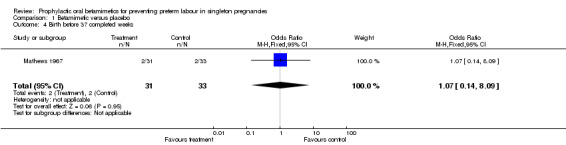
Comparison 1 Betamimetic versus placebo, Outcome 4 Birth before 37 completed weeks.
1.5. Analysis.
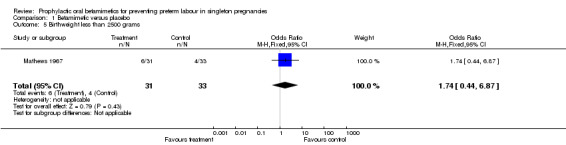
Comparison 1 Betamimetic versus placebo, Outcome 5 Birthweight less than 2500 grams.
1.6. Analysis.
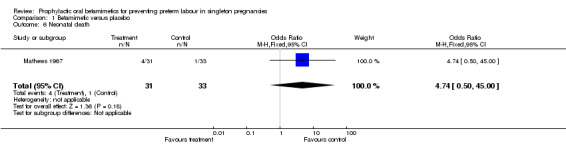
Comparison 1 Betamimetic versus placebo, Outcome 6 Neonatal death.
1.7. Analysis.
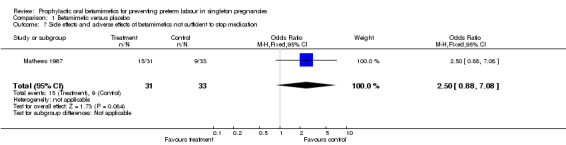
Comparison 1 Betamimetic versus placebo, Outcome 7 Side effects and adverse effects of betamimetics not sufficient to stop medication.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mathews 1967.
| Methods | Performance bias: blinding of participants ‐ yes; blinding of caregiver ‐ yes; blinding of outcome assessment ‐ yes. Attrition bias: 15.6% of participants discontinued medication. Completeness of follow up: yes. | |
| Participants | Country: UK. Number: 31 women in the intervention group, 33 women in the control group. Gestational age at trial entry: between 28 and 32 weeks. | |
| Interventions | Isoxsuprine 30 mg 4 times a day versus placebo. | |
| Outcomes | Incidence of: ‐ birth less than 36 weeks; ‐ neonatal mortality; ‐ birthweight less than 2500 grams. Mean gestational age. Mean birthweight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 1986 | Comparison of preterm delivery rates of asthmatic women routinely taking betamimetics and those on no betamimetics. Observational study not a RCT. |
| Allen 1965 | Trial of isoxsuprine to inhibit acute preterm labour. |
| Briscoe 1966 | Trial tested the effects of oral isoxsuprine in preventing preterm labour in low‐risk women. |
| Hallak 1993 | Trial of the effect of betamimetics on fetal umbilical artery velocimetry in low‐risk pregnancy. |
| Hendricks 1961 | Trial of the effect of isoxsuprine on uterine contractions in women in normal and preterm labour. Observational study not a RCT. |
| Matijevic 2006a | Trial of maintenance tocolytic therapy. |
| Walters 1977 | Trial comparing the effects of oral ritodrine and placebo on preterm delivery rates of primigravid women in whom the internal os of the cervix was one or more fingerbreadths dilated at 28 to 32 weeks' gestation. Whilst cervical change on digital examination was thought at the time this study was conducted to be an accurate predictor of preterm delivery subsequent work has shown that routine digital examination, which is a subjective and non‐specific examination, has a poor positive predictive value and may influence the number of unnecessary hospital admissions or tocolytic use. |
RCT: randomised controlled trial
Contributions of authors
MK Whitworth wrote the first draft of the review and revised the review in response to editorial comments. S Quenby commented on the first and final draft of the review. MK Whitworth is the guarantor of the review.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Mathews 1967 {published data only}
- Mathews DD, Friend JB, Michael CA. A double‐blind trial of oral isoxuprine in the prevention of premature labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1967;74:68‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 1986 {published data only}
- Alexander S, Yudkin P. Betamimetic drugs in the prophylaxis of preterm labour: extent and rationale of their use. British Journal of Obstetrics and Gynaecology 1986;93:891‐2. [DOI] [PubMed] [Google Scholar]
Allen 1965 {published data only}
- Allen H, Short H, Fraleigh D. The use of isoxsuprine in the management of preterm labour. Applied Therapeutics 1965;7:544‐7. [PubMed] [Google Scholar]
Briscoe 1966 {published data only}
- Briscoe CC. Failure of oral isoxsuprine to prevent prematurity. American Journal of Obstetrics and Gynecology 1966;95:885‐6. [DOI] [PubMed] [Google Scholar]
Hallak 1993 {published data only}
- Hallak M, Moise KJ, Lira N, Dorman K, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing (FBM) and body movements (FM): a prospective, randomized, double blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;166:375. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, Lira N, Dorman KF, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: a prospective, randomized, double‐blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;167:1059‐63. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized double‐blind study. American Journal of Obstetrics and Gynecology 1993;168:348. [DOI] [PubMed] [Google Scholar]
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 1993;168:865‐8. [DOI] [PubMed] [Google Scholar]
Hendricks 1961 {published data only}
- Hendricks C, Cibils L, Pose S, Eskes T. The pharmacologic control of excessive uterine activity with isoxsuprine. American Journal of Obstetrics and Gynecology 1961;82(5):1064‐78. [DOI] [PubMed] [Google Scholar]
Matijevic 2006a {published data only}
- Matijevic R, Grgic O, Vasilj O. Ritodrine in oral maintenance of tocolysis after active preterm labor: randomized controlled trial. Croatian Medical Journal 2006;47:25‐31. [PMC free article] [PubMed] [Google Scholar]
Walters 1977 {published data only}
- Walters WAW, Wood C. A trial of oral ritodrine for the prevention of premature labour. British Journal of Obstetrics and Gynaecology 1977;84:26‐30. [DOI] [PubMed] [Google Scholar]
Additional references
Althuisius 1998
- Althuisius S, Dekker G, Hummel P, Bekedam D, Kuik D, Giejn HP. Cervical incompetence prevention randomisation cerclage trial (CIPRACT); effect of therapeutic cerclage with bed rest v bed rest only on cervical length. Ultrasound in Obstetrics & Gynecology 1998;12:312‐7. [DOI] [PubMed] [Google Scholar]
Anotayanonth 2004
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD004352. DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Barden 1980
- Barden TP. Ritodrine hydrochloride: an FDA‐approved tocolytic agent for use in the United States. Part I. Pharmacology, clinical history, administration, side effects and safety. Obstetrics & Gynecology 1980;56:1‐6. [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bienstock 2001
- Bienstock JL, Ural SH, Blakemore K, Pressman EK. University hospital‐based prenatal care decreases rate of preterm delivery and costs, when compared to managed care. Journal of Maternal‐Fetal Medicine 2001;10(2):127‐30. [DOI] [PubMed] [Google Scholar]
Bracci 2006
- Bracci R, Perrone S, Buonocore G. The timing of neonatal brain damage. Biology of the Neonate 2006;90(3):145‐55. [DOI] [PubMed] [Google Scholar]
Costeloe 2000
- Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000;106(4):659‐71. [DOI] [PubMed] [Google Scholar]
da Fonseca 2003
- Fonseca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo‐controlled double‐blind study. American Journal of Obstetrics and Gynecology 2003;188(2):419‐24. [DOI] [PubMed] [Google Scholar]
Dodd 2006
- Dodd JM, Crowther CA, Dare MR, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003927.pub2] [DOI] [PubMed] [Google Scholar]
Gabor 1982
- Gabor H, James MR. Biochemistry and pharmacology of the myometrium and labor: regulation at the cellular and molecular levels. American Journal of Obstetrics and Gynecology 1982;142:225‐37. [DOI] [PubMed] [Google Scholar]
Gyetvai 1999
- Gyetvai K, Hannah ME, Hodnett ED, Ohlsson A. Tocolytics for preterm labor: a systematic review. Obstetrics & Gynecology 1999;94:869‐77. [DOI] [PubMed] [Google Scholar]
Hack 1999
- Hack M. Consideration of the use of health status, functional outcome, and quality‐of‐life to monitor neonatal intensive care practice. Pediatrics 1999;103(1 Suppl E):319‐28. [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Hoffman 1984
- Hoffman HJ, Bakketeig lS. Risk factors associated with the occurrence of preterm birth. Clinical Obstetrics and Gynecology 1984;27(3):539‐52. [DOI] [PubMed] [Google Scholar]
ICROP 1984
- Anonymous. An international classification of retinopathy of prematurity. The committee for the classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [DOI] [PubMed] [Google Scholar]
Kramer 2000
- Kramer M, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. JAMA 2000;284:843‐9. [DOI] [PubMed] [Google Scholar]
Liggins 1972
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50(4):515‐25. [PubMed] [Google Scholar]
Lumley 2003
- Lumley J. Defining the problem: epidemiology of preterm birth. BJOG: an international journal of obstetrics and gynaecology 2003;110(Suppl 20):3‐7. [PubMed] [Google Scholar]
Meis 2003
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha‐hydroxyprogesterone caproate. New England Journal of Medicine 2003;384(24):2379‐85. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Petrou 2001
- Petrou S, Sach T, Davidson L. The long‐term costs of preterm birth and low birth weight: results of a systematic review. Child Care, Health and Development 2001;27:97‐115. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Sciscione 2003
- Sciscione AC, Ivester T, Largoza M, Manley J, Shlossman P, Colmogen GHC. Acute pulmonary edema in pregnancy. Obstetrics & Gynecology 2003;101:511‐5. [DOI] [PubMed] [Google Scholar]
Sosa 2004
- Sosa C, Althabe F, Belizán J, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD003581. DOI: 10.1002/14651858.CD003581.pub2] [DOI] [PubMed] [Google Scholar]
Ugwumadu 2003
- Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 2003;361:983‐8. [DOI] [PubMed] [Google Scholar]
Wood 2005
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, EPICure Study Group. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(2):F134‐F140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamasmit 2005
- Yamasmit W, Chaithongwongwatthana S, Tolosa JE, Limpongsanurak S, Pereira L, Lumbiganon P. Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 3. [Art. No.: CD004733. DOI: 10.1002/14651858.CD004733.pub2] [DOI] [PubMed] [Google Scholar]


