Abstract
Objective
The aim of the study is to compare maternal and neonatal outcomes among patients who are normotensive, hypertensive by Stage I American College of Cardiology-American Heart Association (ACC-AHA) criteria, and hypertensive by American College of Obstetricians and Gynecologists (ACOG) criteria.
Study Design
Secondary analysis of a prospective first trimester cohort study between 2007 and 2010 at three institutions in Baltimore, MD, was conducted. Blood pressure at 11 to 14 weeks’ gestation was classified as (1) normotensive (systolic blood pressure [SBP] <130 mm Hg and diastolic blood pressure [DBP] <80 mm Hg); (2) hypertensive by Stage I ACC-AHA criteria (SBP 130–139 mm Hg or DBP 80–89 mm Hg); or (3) hypertensive by ACOG criteria (SBP ≥140 mm Hg or DBP ≥90 mm Hg). Primary outcomes included preeclampsia, small for gestational age (SGA) neonate, and preterm birth.
Results
Among 3,422 women enrolled, 2,976 with delivery data from singleton pregnancies of nonanomalous fetuses were included. In total, 20.2% met hypertension criteria (Stage I ACC-AHA n = 254, 8.5%; ACOG n = 347, 11.7%). The Stage I ACC-AHA group’s risk for developing preeclampsia was threefold higher than the normotensive group (adjusted relative risk [aRR] 3.70, 95% confidence interval [CI] 2.40–5.70). The Stage I ACC-AHA group had lower preeclampsia risk than the ACOG group but the difference was not significant (aRR 0.87, 95% CI 0.55–1.37). The Stage I ACC-AHA group was more likely than the normotensive group to deliver preterm (aRR 1.44, 95% CI 1.02–2.01) and deliver an SGA neonate (aRR 1.51, 95% CI 1.07–2.12). The Stage I ACC-AHA group was less likely to deliver preterm compared with the ACOG group (aRR 0.65, 95% CI 0.45–0.93), but differences in SGA were not significant (aRR 1.31, 95% CI 0.84–2.03).
Conclusion
Pregnant patients with Stage I ACC-AHA hypertension in the first trimester had higher rates of preeclampsia, preterm birth, and SGA neonates compared with normotensive women. Adverse maternal and neonatal outcomes were numerically lower in the Stage I ACC-AHA group compared with the ACOG group, but these comparisons only reached statistical significance for preterm birth. Optimal pregnancy management for first trimester Stage I ACC-AHA hypertension requires active study.
Keywords: hypertension criteria, preeclampsia, preterm delivery, small for gestational age, chronic hypertension
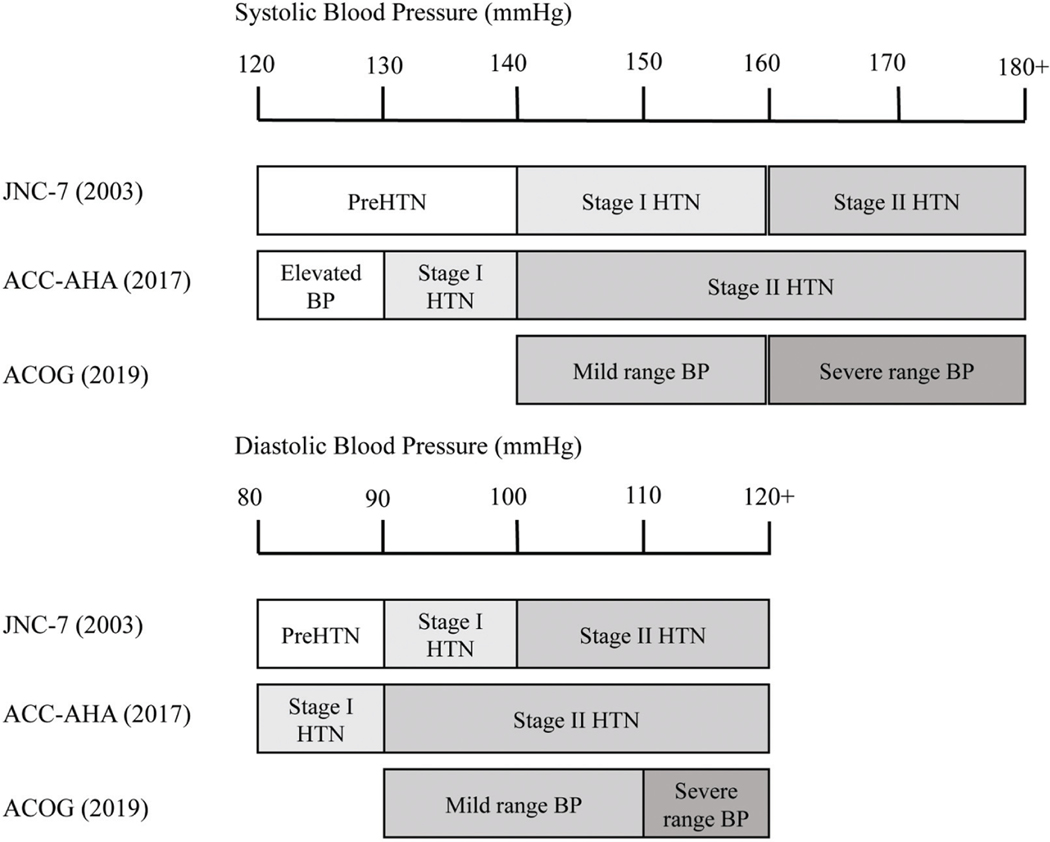
Hypertension is the leading risk factor for global disease burden, and remains the top risk factor for all-cause mortality in the United States.1,2 Recent evidence suggests that blood pressures below thresholds, historically considered hypertensive, still lead to increased risk of cardiovascular disease and death, and trials have suggested benefit to treatment.3,4 In light of these data, in 2017 the ACC-AHA lowered its thresholds for Stage I and Stage II hypertension for the nonpregnant population with the aim to reduce lifetime risk of cardiovascular disease (►Fig. 1).5
Fig. 1.
Differences in hypertension criteria by society. Illustration of differences between Joint National Committee 7, 2017 American College of Cardiology and American Heart Association, and American College of Obstetricians and Gynecologists Guidelines for hypertension classification.
Since then, the ACOG has reaffirmed its single diagnostic threshold for hypertension in pregnancy at a systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, which is the same diagnostic threshold as Stage II hypertension in ACC-AHA guidelines. Currently, implications of the lower Stage I ACC-AHA classification (of SBP 130–139 mm Hg or DBP 80–89 mm Hg) on obstetric outcomes and potential benefit of intervention remain unclear.6 Considering the consistent relationship between first trimester blood pressure and adverse outcome attributable to placental disease, identifying blood pressure risk thresholds in pregnancy is of considerable importance.7–9 Recent investigations on the relevance of the new hypertension criteria to pregnancy suggest that pregnant women with Stage I hypertension by ACC-AHA criteria experience more adverse outcomes compared with normotensive women. However, these studies have been conducted in populations of low-risk, nulliparous women, who are not universally representative, and have lacked comparison groups of women with higher baseline blood pressures.10,11 Other investigations have been derived by single center studies, relatively noncontemporary data, and populations that may not reflect US demographics.12–14 We sought to characterize outcomes in pregnant women with first trimester stage ACC-AHA Stage I hypertension in a nonselected, moderate-risk, contemporary US cohort.
Materials and Methods
Study Design
This was a secondary analysis of a prospective preeclampsia cohort study conducted at three institutions in Baltimore, Maryland between 2007 and 2010 intended to identify first trimester predictors of preeclampsia.15 This study was approved by the Institutional Review Boards of the University of Maryland School of Medicine, Mercy Medical Center, Medstar Research Institute, and the Johns Hopkins University School of Medicine. Women presenting for a first trimester screen were offered enrollment at 11 to 14 weeks’ gestation and gestational age was confirmed by ultrasound examination. After informed written consent was obtained, a single blood pressure measurement was collected in standardized fashion as previously described; in summary, single blood pressure measurements were taken by trained staff after 5 minutes of maternal rest with patients in a seated position and the arm at the level of the heart.15 The Dinamap Pro1000 V3 automated sphygmomanometer was used with an appropriate cuff for patient arm circumference and was calibrated every 6 months in accordance with the Association for the Advancement of Medical Instrumentation guidelines. We classified enrollment blood pressure as either (1) normotensive (SBP < 130 mm Hg and DBP < 80 mmHg); (2) hypertensive by Stage I ACC-AHA criteria (SBP 130–139 mm Hg or DBP 80–89 mm Hg); or (3) hypertensive by ACOG criteria (SBP ≥140 mm Hg or DBP ≥90 mm Hg). Twenty-two women on antihypertensive medications at the time of enrollment were categorized into the ACOG group regardless of enrollment blood pressure.
Outcomes
Pregnancy outcomes were collected by research staff and verified by source documentation. Adverse pregnancy outcomes were evaluated and included preeclampsia, small for gestational age (SGA) neonate, and preterm delivery. Pre-eclampsia was defined as new-onset or worsening proteinuria (defined as 2+ or greater on point of care urinalysis) and maternal SBP ≥140 mm Hg or DBP ≥90 mm Hg on two separate occasions, 6 or more hours apart, after 20 weeks’ gestation. Among women in the ACOG group, superimposed preeclampsia was defined using the parameters of worsening blood pressure and development of proteinuria, as above. In this analysis, the term preeclampsia additionally includes women with superimposed preeclampsia. Secondary outcomes included preeclampsia requiring magnesium, pre-eclampsia requiring delivery before 34 weeks’ gestation, and preterm birth before 34 weeks’ gestation. Preeclampsia requiring magnesium sulfate for delivery served as a surrogate for severe maternal disease. Disease severity was additionally evaluated with complete blood count and comprehensive metabolic panel. SGA was defined as birthweight below the 10th percentile at time of delivery by sea level standards.16
Statistical Analysis
Participant demographics, enrollment characteristics, and adverse outcomes were analyzed in a univariate fashion. These variables were stratified by blood pressure criteria as outlined above, using a nonparametric test for trend for comparisons across the three categories17 and Kruskal–Wallis tests for continuous variables and chi-squared tests for binary or categorical variables when compared across two categories. Logistic regression models were used to generate adjusted correlations between the categorical hypertensive variable and outcome variable, controlling for potential confounding factors, identified a priori. The factors identified were age, race, parity, prior preeclampsia, body mass index, gestational age at time of blood pressure measurement, use of aspirin during pregnancy, and presence of autoimmune disease or pregestational diabetes. Odds ratios were converted to relative risks for ease of interpretation by calculating the equivalent relative risk averaged over the entire cohort.18 Additionally, the differences between the Stage I ACC-AHA and ACOG groups were calculated from the model coefficients, with standard errors estimated using the delta method. Due to minimal (only 18 [<1%] of body mass index values) missing data, mean imputation was used to impute missing values. No other variables had missing values. All analyses were performed in Stata/IC, Version 15.1 (StataCorp, College Station, TX), with a two-sided α level of 0.05 prespecified as statistically significant.
Results
A total of 2,976 participants with singleton pregnancies carrying a nonanomalous, genetically normal fetus and with complete delivery data were included in this analysis. The median maternal age of the cohort was 30 years (24–35, 25th–75th percentile) and median enrollment gestational age was 12 weeks and 4 days (12 + 1–13 + 0, 25th–75th percentile). In total, 1,504 women (50.5%) were black and 761 (25.6%) were multiparous; 2,375 (79.8%) women were normotensive at enrollment while 254 (8.5%) had Stage I hypertension by ACC-AHA criteria and 347 (11.7%) had hypertension by ACOG criteria (►Table 1).
Table 1.
Baseline clinical characteristics, stratified by blood pressure at the time of first trimester screen
| Overall (N = 2,976) | Normotensive (N = 2,375) | Stage I ACC-AHA (N = 254) | ACOG (N = 347) | p-Value(overall) | P (Stage I ACC-AHA vs. ACOG) | |
|---|---|---|---|---|---|---|
| Median (25th-75th percentile) or n (%) | ||||||
| Baseline characteristics | ||||||
| Age (y) | 30 (24–35) | 29 (24–35) | 31 (25–36) | 31 (26–36) | <0.001 | 0.30 |
| Gestational age at time of enrollment (weeks + days) | 12 + 4 (12 + 1−13 + 0) |
12 + 4 (12 + 2−13 + 0) |
12 + 3 (12 + 1−13 + 0) |
12 + 4 (12 + 1−13 + 0 |
0.16 | 0.34 |
| Race/ethnicity | <0.001 | 0.25 | ||||
| Asian | 149 (5.0) | 135 (5.7) | 8(3.1) | 6(1.7) | ||
| Black | 1,504 (50.5) | 1,122 (47.2) | 157 (61.8) | 225 (64.8) | ||
| Hispanic | 35(1.2) | 26 (1.1) | 2 (0.8) | 7 (2.0) | ||
| White/Other | 1,288 (43.3) | 1,092 (46.0) | 87 (34.3) | 109 (31.4) | ||
| Multiparous | 761 (25.6) | 550 (23.2) | 74 (29.1) | 137 (39.5) | <0.001 | 0.009 |
| Pregestational diabetes mellitus | 120 (4.0) | 41 (1.7) | 15(5.9) | 64 (18.4) | <0.001 | <0.001 |
| Autoimmune condition | 32 (1.1) | 21 (0.9) | 2 (0.8) | 9 (2.6) | 0.009 | 0.10 |
| History of preeclampsia | 147 (4.9) | 58 (2.4) | 18(7.1) | 71 (20.5) | <0.001 | <0.001 |
| Prepregnancy body mass index (kg/m2) | 25.8 (22.2–31.2) |
24.9 (21.8–29.5) |
30.0 (25.2–37.6) |
32.6 (26.3–39.8) |
<0.001 | 0.008 |
| Initiation of aspirin therapy | <0.001 | <0.001 | ||||
| Never | 2,375 (79.8) | 1,948 (82.0) | 205 (80.7) | 222 (64.0) | ||
| Prior to 11 wk | 166 (5.6) | 102 (4.3) | 16 (6.3) | 48 (13.8) | ||
| Between 11 and 14 wk | 351 (11.8) | 259 (10.9) | 25 (9.8) | 67 (19.3) | ||
| After 14 wk | 84 (2.8) | 66 (2.8) | 8(3.1) | 10 (2.9) | ||
With increasing enrollment blood pressure, demographics by race changed from a higher proportion of White and Asian women in the normotensive group to a higher proportion of Black women in the Stage I ACC-AHA and ACOG groups (47.2% normotensive vs. 61.8% Stage I ACC-AHA vs 64.8% ACOG). Prevalence of multiparty, obesity, use of aspirin during pregnancy, as well as history of preeclampsia, preexisting diabetes, and autoimmune disease increased with higher first trimester blood pressures when we compared the normotensive, Stage I ACC-AHA, and ACOG groups (►Table 1). Rate of cesarean section was highest in the ACOG group. Among participants diagnosed with preeclampsia, liver enzymes, renal function, and uric acid levels were similar among groups and within the normal range (data not shown). Platelet count was also within normal range across groups but was significantly higher in the ACOG group.
We observed an increasing proportion of adverse pregnancy outcomes attributable to high blood pressure with increasing blood pressure categories (►Table 2). The normotensive group was significantly less likely than the Stage I ACC-AHA group to have preeclampsia (3.0 vs. 11.4%, p < 0.001), requiring magnesium (2.1 vs. 8.3%, p < 0.001), and experience preterm birth (9.8 vs. 14.2%, p < 0.001). We continued to observe this trend in our higher blood pressure categories as the Stage I ACC-AHA group was less likely than the ACOG group to have preeclampsia (11.4 vs. 16.7%, p = 0.07), requiring magnesium (8.3 vs. 14.7%, p = 0.02), and experience preterm birth (14.6 vs. 26.8%, p < 0.001).
Table 2.
Pregnancy outcomes, stratified by blood pressure at the time of first trimester screen
| Overall (N = 2,976) | Normotensive (N = 2,375) | Stage I ACC-AHA (N = 254) | ACOG (N = 347) | P (overall) | P (Stage I ACC-AHA vs. ACOG) | |
|---|---|---|---|---|---|---|
| Median (25th-75th percentile) or n (%) | ||||||
| Outcomes | ||||||
| Preeclampsia | 159 (5.3) | 73 (3.0) | 29 (11.4) | 58 (16.7) | <0.001 | 0.07 |
| Preeclampsia requiring magnesium | 123 (4.1) | 51 (2.1) | 21 (8.3) | 51 (14.7) | <0.001 | 0.02 |
| Preeclampsia, with onset before or at 34 wk, 0 d-gestation | 30 (1.0) | 12(0.5) | 3(1.2) | 15(4.3) | <0.001 | 0.03 |
| Preeclampsia, with onset after 34wk, 0-d-gestationa | 129 (4.4) | 60 (2.5) | 26 (10.4) | 43 (13.0) | <0.001 | 0.34 |
| Birthweight less than the 10th percentile | 312 (10.5) | 232 (9.8) | 36 (14.2) | 44 (12.7) | 0.03 | 0.48 |
| Preterm birth (<37-wk gestation) | 354 (11.9) | 224 (9.4) | 37 (14.6) | 93 (26.8) | <0.001 | <0.001 |
| Preterm birth (<34-wk gestation) | 121 (4.1) | 71 (3.0) | 15(5.9) | 35 (10.1) | <0.001 | 0.07 |
| Preterm birth (between 34 and 37-wk gestation)b | 233 (8.2) | 153 (6.6) | 22 (9.2) | 58 (18.6) | <0.001 | 0.002 |
| 5-min APGAR score <6 | 54 (1.8) | 35(1.5) | 9 (3.6) | 10 (2.9) | 0.02 | 0.64 |
| NICU admission | 326 (11.0) | 209 (8.8) | 38 (15.0) | 79 (22.8) | <0.001 | 0.02 |
| Delivery method | <0.001 | 0.04 | ||||
| Spontaneous vaginal delivery | 1,957 (65.8) | 1,615 (68.0) | 159 (62.6) | 183 (52.7) | ||
| Cesarean Section | 915 (30.8) | 673 (28.3) | 86 (33.9) | 156 (45.0) | ||
| Operative vaginal delivery | 94 (3.2) | 80 (3.4) | 8(3.1) | 6(1.7) | ||
| TAB or SAB | 9 (0.3) | 6 (0.3) | 1 (0.4) | 2 (0.6) | ||
Abbreviations: ACC-AHA, American College of Cardiology-American Heart Association; ACOG, American College of Obstetricians and Gynecologists; NICU, neonatal intensive care unit; SAB, spontaneous abortion; TAB, therapeutic abortion.
Excluding patients with onset of preeclampsia before 34 wk.
Excluding patients delivered before 34 wk.
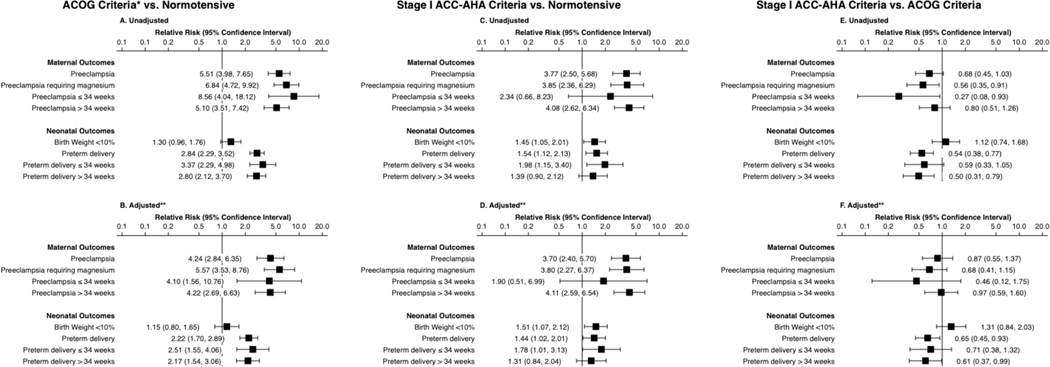
In multivariate analyses, the largest differences in risk were observed when comparing the normotensive group and the ACOG group, showing significantly elevated risk in both unadjusted and adjusted analyses for all maternal and neonatal outcomes except for delivery of an SGA neonate (adjusted relative risk [aRR)] 1.15, 95% confidence interval [CI] 0.80–1.65; ►Fig. 2).
Fig. 2.
Unadjusted and adjusted associations between first trimester blood pressure and outcomes. Unadjusted (A, C, E) and covariate-adjusted (B, D, F) associations between patient blood pressure at the time of first trimester screen and outcomes. Panels A and B compare patients hypertensive by ACOG criteria to normotensive patients. Panels C and D compare patients hypertensive only by Stage I ACC-AHA criteria to normotensive patients. Panels E and F compare hypertensive only by Stage I ACC-AHA criteria to patients hypertensive by ACOG criteria. ACC-AHA, American College of Cardiology-American Heart Association; ACOG, American College of Obstetricians and Gynecologists.
We then compared the normotensive group to the Stage I ACC-AHA group. In unadjusted analyses, the Stage I ACC-AHA group appeared at elevated risk of both adverse maternal and neonatal outcomes. In adjusted analyses, the Stage I ACC-AHA group was more likely to have preeclampsia (aRR 3.70, 95% CI 2.40–5.70) and preeclampsia requiring magnesium (aRR 3.80, 95% CI 2.27–6.37). The Stage I ACC-AHA group was also more likely to deliver preterm (aRR 1.44, 95% CI 1.02–2.01) and deliver an SGA neonate (aRR 1.51, 95% CI 1.07–2.12).
Finally, we compared the Stage I ACC-AHA group to the ACOG group. Unadjusted analyses suggested that the Stage I ACC-AHA group had reduced risk of preeclampsia requiring magnesium and preeclampsia before 34 weeks’ gestation, as well as reduced risk of preterm and late preterm delivery. In adjusted analyses, differences between the two groups were attenuated. Statistically significant differences remained for preterm delivery (aRR 0.65, 95% CI 0.45–0.93) and late preterm delivery (aRR 0.61, 95% CI 0.37–0.99). Although numeric risk remained lower for the Stage I ACC-AHA group, there was no longer a statistically significant difference in preeclampsia requiring magnesium (aRR 0.68, 95% CI 0.41–1.15) or preeclampsia before 34 weeks’ gestation (aRR 0.46, 95% CI 0.12–1.75). In neither unadjusted nor adjusted analyses was the overall preeclampsia incidence significantly different between groups.
Discussion
Compared with women with lower blood pressures, adverse pregnancy outcomes related to hypertensive disorders of pregnancy are increased for women who meet first trimester blood pressure criteria for Stage I ACC-AHA hypertension and are most frequent when ACOG/Stage II ACC-AHA hypertension criteria are met. Although women with milder (Stage I ACC-AHA) hypertension had numerically lower adverse outcomes than more severe (ACOG/Stage II ACC-AHA) hypertension, adjusted analysis revealed a significant difference only for preterm birth.
Findings Related to Other Studies
Recently published multicenter studies in healthy populations have shown that pregnant women who meet 2017 Stage I ACC-AHA criteria early in pregnancy are at increased risk of adverse pregnancy outcomes. A secondary analysis of a multicenter cohort study of healthy, nulliparous women enrolled in the first trimester found that women with Stage I hypertension had elevated risk of gestational hypertension and preeclampsia compared with women with ACC-AHA “elevated” blood pressures (SBP 120–129) and to women normotensive by ACC-AHA criteria.11 Another secondary analysis of a randomized controlled trial of aspirin versus placebo in healthy, nulliparous women conducted in the 1990s with Stage I hypertensive blood pressure documented between 13 and 25 weeks found that these women are at increased risk of preeclampsia, diabetes mellitus, and preterm birth.10 Older studies have used previous hypertension parameters, including previous JNC-7 criteria, for the classification of elevated blood pressures and have shown similar results.12,14 Our analysis builds on these studies by providing contemporary data from a well-characterized, moderate-risk, urban cohort of patients in which the definition of Stage I hypertension is consistent with new ACC-AHA guidelines and women with first trimester blood pressures in both higher and lower strata are included. These are women most likely to be intervened upon by the healthcare providers during pregnancy and they may be the one who could derive the most benefits from interventions that could reduce the risk of preeclampsia, preterm birth, and SGA neonate.
Clinical and Research Implications
Chronic hypertension is a known risk factor for the development of preeclampsia and associated morbidity,19–22 although many cases of preeclampsia can occur in nulliparous women without major comorbidities. For pregnant women who have chronic hypertension based on ACOG criteria, pregnancy surveillance includes antenatal fetal testing, additional sonograms to evaluate for growth restriction, and induction of labor at earlier gestational ages compared with nonhypertensive counterparts. Future prospective studies are needed to determine whether women with Stage I ACC-AHA hypertension require a similar level of clinical surveillance.
Furthermore, the optimal blood pressure management in pregnancy remains controversial due to concern for iatrogenic uteroplacental insufficiency leading to a growth-restricted neonate without demonstrated benefit to the mother.15,23,24 Currently, ACOG recommends initiation of antihypertensive medications for pregnant women when blood pressures are severe and consistently elevated, i.e., SBP ≥160 mm Hg and/or DBP ≥110 mm Hg.6 Observational studies suggest that the risk of preeclampsia and delivery of SGA neonate is lower for women who maintain or improve their blood pressures throughout pregnancy8,25; however, randomized trials do not show the same benefit.26,27 Previous studies have been underpowered for women enrolled in the first trimester, during which the inflammatory and ischemic changes that occur from abnormal placentation are implicated in pre-eclampsia and fetal growth restriction,28 and have not defined specific treatment targets for women enrolled at this gestational age. Accordingly, further research into tight blood pressure control initiated in the first trimester is warranted and ongoing (Chronic Hypertension and Pregnancy, NCT02299414).
Strengths and Limitations
As a secondary analysis of a prospectively designed study for preeclampsia prediction in the first trimester, this study has several strengths. The prospective nature allowed for collection of, and adjustment for, multiple potential confounding factors that predispose women to preeclampsia. Blood pressures were measured in a standardized and reproducible manner, and were measured at a similar gestational age (11–14 weeks), with gestational ages verified at the time of enrollment. Our study includes a diverse cohort of women that is demographically representative and comparable to an urban population at elevated risk of adverse pregnancy outcomes. Finally, our study enrolled women with chronic hypertension by ACOG criteria, which allowed us to compare the Stage I ACC-AHA group to women with both higher and lower first trimester blood pressures. ACOG-defined chronic hypertension has been an exclusion criterion of previous, similar studies.10,11,14,29
This study has some limitations. First, our analysis used a single first trimester blood pressure measurement for stratification of this cohort, although both ACC-AHA and ACOG require two or more elevated blood pressures to qualify for the diagnosis of hypertension.5,6 Participants were fitted for blood pressure cuff size without formal measurement of arm circumference; however, this most closely resembles actual practice. There are factors that may have resulted in uniform underestimation of the incidence of preeclampsia among these women by current criteria, including that diagnosis of preeclampsia no longer requires proteinuria for diagnosis30 and this study lacks data on incidence of postpartum preeclampsia after discharge from delivery hospitalization. The number of women in this cohort who would have preeclampsia by current criteria is unable to be calculated secondary to the lack of uniform acquisition of laboratory studies of women with elevated blood pressures. Generalizability of this study may be limited to populations with similar demographics, and the cohort does under-represent groups including Native American, Hispanic, and Asian patients compared with the US population.
Conclusion
Expanded diagnostic criteria for hypertension, if applied to pregnant women in the first trimester of pregnancy, identify a population at intermediate risk for adverse pregnancy outcomes compared with women with lower blood pressures and women who meet ACOG’s current criteria for hypertension. Further investigation of this intermediate population and determination of optimal pregnancy characterization, surveillance, and management is warranted.
Key Points.
Women with first trimester American College of Cardiology-American Heart Association (ACC-AHA) Stage I hypertension were more likely to develop preeclampsia, deliver preterm, and deliver a small-for-gestational age neonate than normotensive women.
Women with first trimester American College of Obstetricians and Gynecologists (ACOG) hypertension (consistent with stage II ACC-AHA hypertension) had the highest numeric rate of adverse outcomes; however, compared with Stage I ACC-AHA hypertension, there was only statistically significant difference for preterm delivery.
The risk profile for pregnant women with Stage I ACC-AHA hypertension and women with hypertension by conventional ACOG criteria may be more similar than previously understood.
Funding
K.C.D. and B.L.S. received funding from a Kelly Resident Research Award from the Department of Gynecology and Obstetrics, Johns Hopkins School of Medicine. A.J.V. received funding through the Eunice Kennedy Shriver National Institute of Child Health & Human Development Building Interdisciplinary Research in Women’s Health (BIRCWH) Award (K12-HD085845) and the Johns Hopkins University School of Medicine Robert E. Meyerhoff Professorship Award.
Footnotes
Note
A preliminary version of this report was presented in oral form at the 39th Annual Society for Maternal-Fetal Medicine Annual Pregnancy Meeting (February 11–16, 2019 in Las Vegas, NV).
Conflict of Interest
None declared.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380 (9859):2224–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6(04):e1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360 (9349):1903–1913 [DOI] [PubMed] [Google Scholar]
- 4.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373(22):2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018; 71(06):1269–1324 [DOI] [PubMed] [Google Scholar]
- 6.ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol 2019;133(01):e26–e50 [DOI] [PubMed] [Google Scholar]
- 7.Scholten RR, Hopman MT, Sweep FC, et al. Co-occurrence of cardiovascular and prothrombotic risk factors in women with a history of preeclampsia. Obstet Gynecol 2013;121(01):97–105 [DOI] [PubMed] [Google Scholar]
- 8.Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R study. Eur Heart J 2011;32(24):3088–3097 [DOI] [PubMed] [Google Scholar]
- 9.Baschat AA. First-trimester screening for pre-eclampsia: moving from personalized risk prediction to prevention. Ultrasound Obstet Gynecol 2015;45(02):119–129 [DOI] [PubMed] [Google Scholar]
- 10.Sutton EF, Hauspurg A, Caritis SN, Powers RW, Catov JM. Maternal outcomes associated with lower range stage 1 hypertension. Obstet Gynecol 2018;132(04):843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauspurg A, Parry S, Mercer BM, et al. Blood pressure trajectory and category and risk of hypertensive disorders of pregnancy in nulliparous women. Am J Obstet Gynecol 2019;221(03):277. e1–277.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He D, Wu S, Zhao H, Zheng Z, Zhang W. High normal blood pressure in early pregnancy also contribute to early onset pre-eclampsia and severe preeclampsia. Clin Exp Hypertens 2018;40 (06):539–546 [DOI] [PubMed] [Google Scholar]
- 13.Rosner JY, Gutierrez M, Dziadosz M, et al. Prehypertension in early pregnancy: what is the significance? Am J Perinatol 2017;34(02): 117–122 [DOI] [PubMed] [Google Scholar]
- 14.Black MH, Zhou H, Sacks DA, et al. Prehypertension prior to or during early pregnancy is associated with increased risk for hypertensive disorders in pregnancy and gestational diabetes. J Hypertens 2015;33(09):1860–1867, discussion 1867 [DOI] [PubMed] [Google Scholar]
- 15.Baschat AA, Magder LS, Doyle LE, Atlas RO, Jenkins CB, Blitzer MG. Prediction of preeclampsia utilizing the first trimester screening examination. Am J Obstet Gynecol 2014;211(05): 514.e1–514.e7 [DOI] [PubMed] [Google Scholar]
- 16.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol 1976;126(05):555–564 [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4(01): 87–90 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol 2010;63(01):2–6 [DOI] [PubMed] [Google Scholar]
- 19.Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016;353:i1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol 2002;100(02):369–377 [DOI] [PubMed] [Google Scholar]
- 21.Ankumah NE, Sibai BM. Chronic hypertension in pregnancy: diagnosis, management, and outcomes. Clin Obstet Gynecol 2017;60 (01):206–214 [DOI] [PubMed] [Google Scholar]
- 22.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014;348:g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su CY, Lin HC, Cheng HC, Yen AM, Chen YH, Kao S. Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PLoS One 2013;8(02):e53844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meidahl Petersen K, Jimenez-Solem E, Andersen JT, et al. Beta-blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2012;2(04):e001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block-Abraham DM, Adamovich D, Turan OM, Doyle LE, Blitzer MG, Baschat AA. Maternal blood pressures during pregnancy and the risk of delivering a small-for-gestational-age neonate. Hypertens Pregnancy 2016;35(03):350–360 [DOI] [PubMed] [Google Scholar]
- 26.Magee LA, Singer J, von Dadelszen P; CHIPS Study Group. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 2015;372(24):2367–2368 [DOI] [PubMed] [Google Scholar]
- 27.Pels A, Mol BWJ, Singer J, et al. ; CHIPS Study Group. Influence of gestational age at initiation of antihypertensive therapy: secondary analysis of CHIPS trial data (Control of Hypertension in Pregnancy Study). Hypertension 2018;71(06):1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008;294(02):H541–H550 [DOI] [PubMed] [Google Scholar]
- 29.Hedderson MM, Darbinian JA, Sridhar SB, Quesenberry CP. Prepregnancy cardiometabolic and inflammatory risk factors and subsequent risk of hypertensive disorders of pregnancy. Am J Obstet Gynecol 2012;207(01):68.e1–68.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019;133(01):e1–e25 [DOI] [PubMed] [Google Scholar]