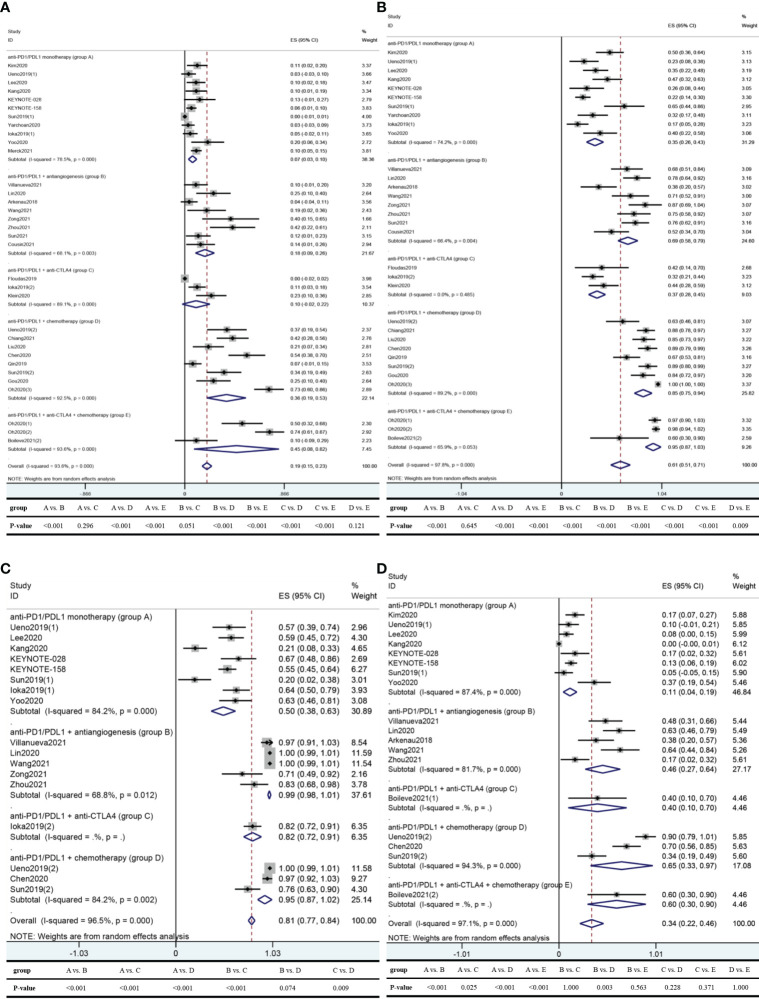
Figure 4.
Pooled results of ORR, DCR, any-grade AEs, and grade 3–4 AEs in total and by medication regimen subgroup. (A) ORR; (B) DCR; (C) any-grade AEs; (D) grade 3–4 AEs. Five studies had more than one subgroup of interest. Specifically, patients were allocated to the nivolumab group [Ueno2019(1)] or the nivolumab/GemCis group [Ueno2019(2)] in the Ueno2019 study; the PD1 inhibitor monotherapy group [Sun2019(1)] or the PD1 inhibitor plus chemotherapy group [Sun2019(2)] in the Sun2019 study; the durvalumab group [Ioka2019(1)] or the durvalumab/tremelimumab group [Ioka2019(2)] in the Ioka2019 study; the biomarker group [receiving durvalumab/tremelimumab with GemCis, Oh2020(1)], the durvalumab/tremelimumab with GemCis group [Oh2020(2)], or the durvalumab with GemCis group [Oh2020(3)] in the Oh2020 study; the durvalumab/tremelimumab group [Boileve2021(1)] or the durvalumab/tremelimumab with paclitaxel group [Boileve2021(2)] in the Boileve2021 study. Note: Heterogeneity across studies was evaluated by the Cochran Q chi-square test and I 2 statistic, with P <0.1 for the Q test deemed to have high heterogeneity and I 2 >50% regarded as an indicator of moderate-to-high heterogeneity. If separate verdicts from the Q test and I 2 statistic were at opposite poles, we would give priority to the conclusion from the I 2 statistic since the former is proverbially underpowered to detect heterogeneity. Differences between groups were tested by the chi-square test using IBM SPSS Statistics 22.0, with two-sided P-value <0.05 considered significant. ORR, objective response rate; DCR, disease control rate; AEs, adverse events; ES, effect size; CI, confidence interval; PD1, programmed cell death protein 1; PDL1, programmed cell death ligand 1; CTLA4, cytotoxic T lymphocyte antigen 4; GemCis, gemcitabine + cisplatin.