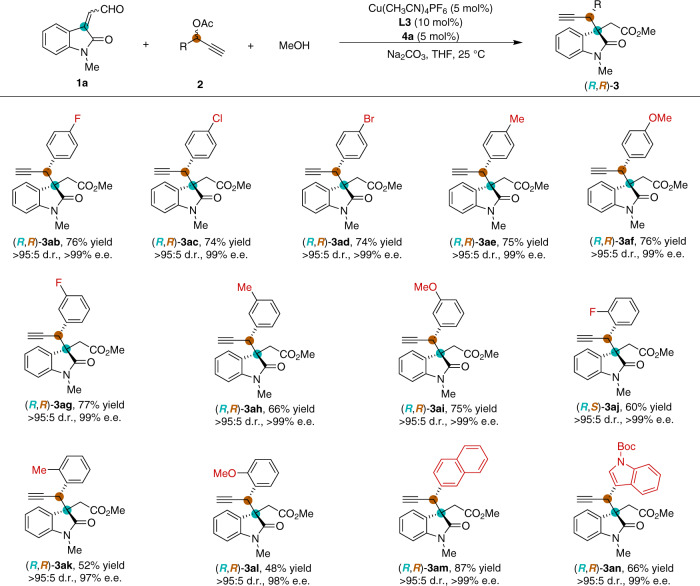
Fig. 3. Substrate scope of propargylic acetates.
Reaction conditions: Cu(CH3CN)4PF6 (5 mol%) and pyridine bis(oxazoline) ligand L3 (10 mol%) were stirred in THF (0.5 mL) at 25 °C for 1 h, then NHC precatalyst 4a (5 mol%), 1a (0.15 mmol), 2 (0.1 mmol), MeOH (0.5 mmol), Na2CO3 (0.1 mmol) and THF (0.5 mL) were added to the reaction mixture and stirred for 12 h under N2. Diastereomeric ratio (d.r.) was determined by 1H NMR spectroscopic analysis. Isolated yields. The enantiomeric excess (e.e.) was determined by HPLC.