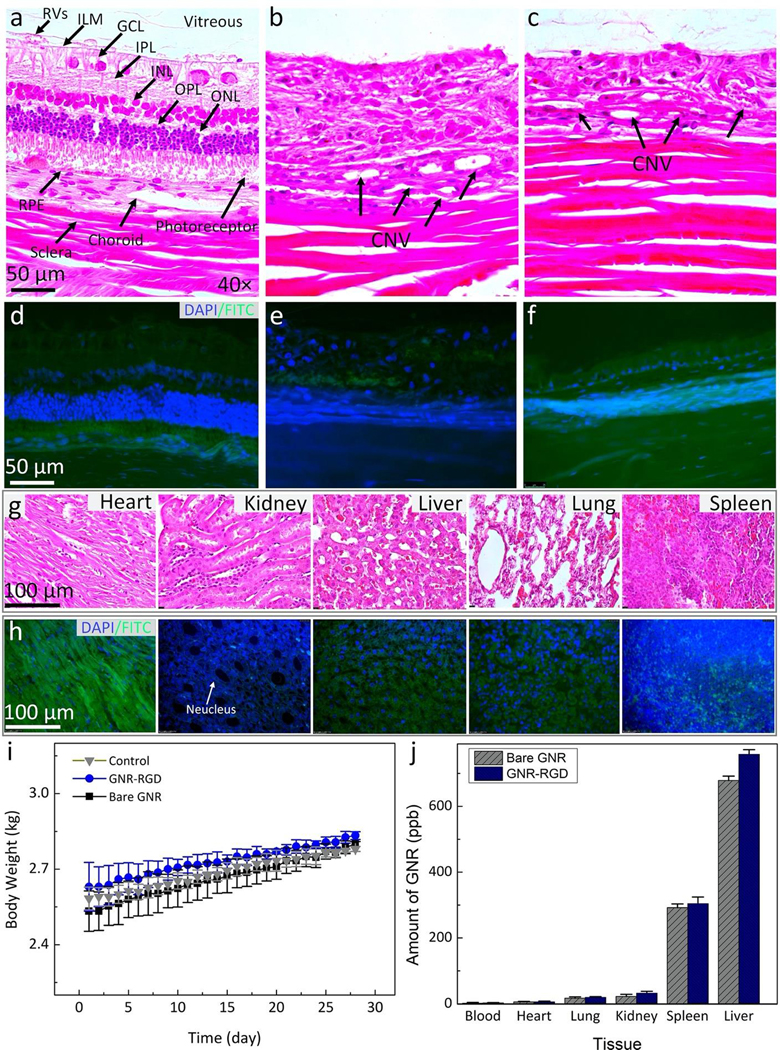
Figure 10. Histological analysis and TUNEL assay after GNR injection in rabbits:
Hematoxylin and eosin (H&E) images of the control (a), laser-induced CNV model (b), and subretinal injection of VEGF-165 (c). The control image shows different layers of the retina such as retinal vessels (RVs), internal limiting membrane (ILM), ganglion cells layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptors, retinal pigment epithelium (RPE), choroid, and sclera. The treated tissues show evidence of the development CNV (black arrows). In addition, a significant change in retinal thickness was observed. However, there were no specific changes in cell morphology related to the administration of gold nanoparticles. (d–f) TUNEL staining of treated tissues with GNR with different groups. The green fluorescent color indicates the TUNEL-positive cells detected in each group. Blue fluorescent color represents cell nuclei stained with DAPI. (g–h) H&E and TUNEL staining of different organs (i.e., heart, kidney, liver, lung, and spleen). There is no tissue disorganization observed on H&E and no TUNEL-positive cells detected on these samples, confirming that GNR-RGD induced minimal systemic toxicity. (i) A graph of body weight measured from three treated groups (control, treated with bare GNR, and GNR-RGD). (j) Biodistribution of GNR in organs post administration of GNR and GNR-RGD. Most GNR accumulated in the spleen and liver.