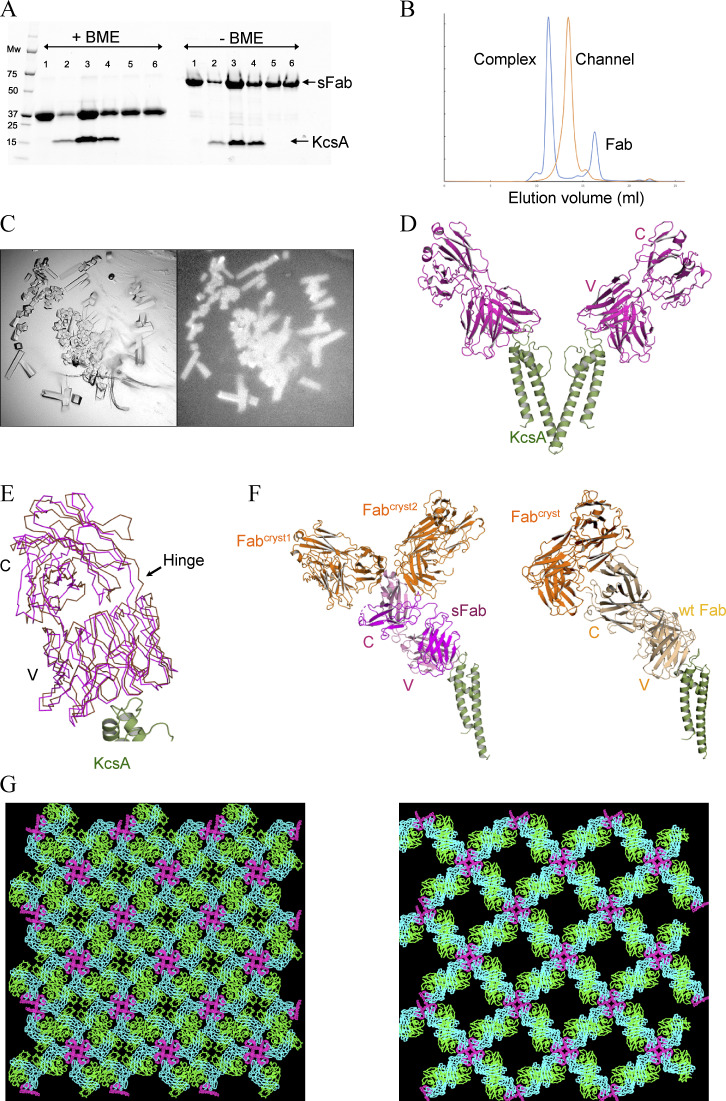
Figure 2.
Expression, purification, and structural determination of the sFab–KcsA complex. (A) An affinity pulldown SDS-PAGE showing the association between KcsA and sFab with (left) and without (right) β-mercaptoethanol (BME). Lane 1: sFab, lane 2–6: sFab–KcsA complex; excess fab is shown in lane 5 and 6 from SEC. (B) Comparison of the analytical size-exclusion chromatograms for the apo (orange) and complex form of KcsA with sFab (blue), with ∼2 ml shift of the elution volume to the left upon complex formation. (C) Crystals of the sFab–KcsA complex visualized under visible (left) and UV (right) light microscope obtained at room temperature after 24 h. (D) The crystal structure of sFab–KcsA where the sFab (magenta) binds at the turret loop of KcsA (green), showing two diagonally opposing monomers for clarity (PDB accession no. 7MDJ). (E) Crystal lattice packing of the sFab–KcsA, structural analysis of the sFab (magenta), and wtFab (gold) showing the positional deviation around the constant domain. (F) Comparison of the interaction between Fabs in crystallographic symmetry mates (Fabcryst, in orange) showing the asymmetric unit of sFab (left) and wtFab (right). (G) Comparison of the crystal lattice from the new sFab–KcsA on the left with that from wtFab–KcsA (PDB accession no. 1K4C) on the right.