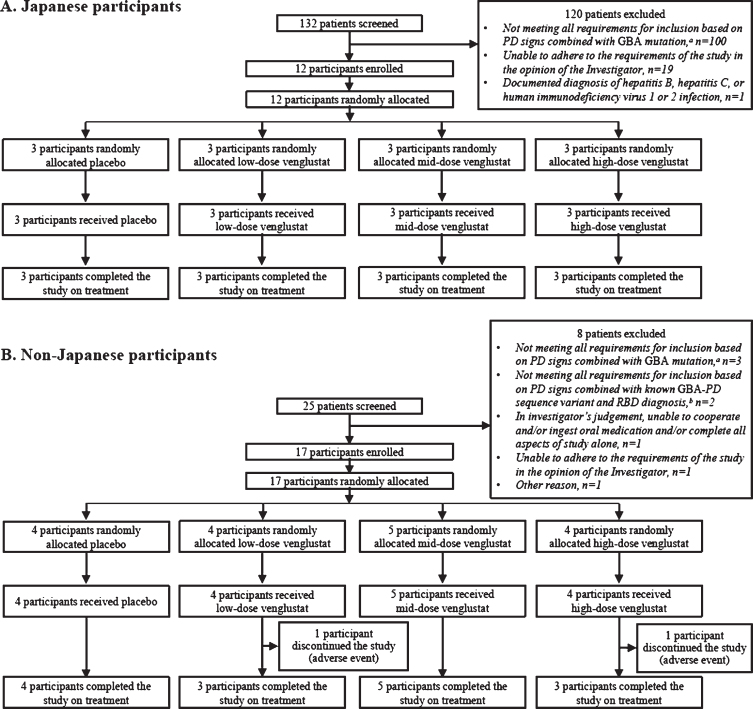
Fig. 1.
CONSORT diagram: disposition of participants enrolled in Part 1 of the MOVES-PD trial. Two non-Japanese participants permanently discontinued the study due to adverse events after receiving venglustat and post week 4 (one participant [low dose] discontinued due to confusional state, and one participant [high dose] due to a panic attack). aInclusion criterion: male or female patients with a diagnosis of PD (with at least two of the following signs: resting tremor, postural instability, akinesia/hypokinesia, and muscle rigidity) and who are heterozygous carriers of a GBA mutation. bInclusion criterion: patients carrying known sequence variants associated with GBA-PD, in addition to having a diagnosis of PD (with at least two of the following signs: resting tremor, postural instability, akinesia/hypokinesia, or muscle rigidity), must also have a diagnosis of RBD confirmed by historically documented polysomnography or by RBD screening questionnaire. GBA, glucocerebrosidase (glucosylceramidase beta) gene; PD, Parkinson’s disease; RBD, rapid eye movement sleep behavior disorder.