Abstract
Background
The watershed in Asubpeeschoseewagong Netum Anishinabek (Grassy Narrows First Nation) territory has been contaminated by mercury (Hg) since 1962, resulting in very high Hg concentrations in fish, central to the community’s culture, traditions, economy and diet. Biomarkers of Hg exposure (umbilical cord blood and hair/blood samples), monitored between 1970 and 1997, decreased over time. A recent Grassy Narrows Community Health Assessment (GN-CHA) survey included current symptoms of nervous system dysfunction. The present study aimed to cluster self-reported symptoms and examine their associations with past Hg exposure.
Methods
The GN-CHA included 391 adults. Symptom clustering used a two-step segmentation approach. Umbilical cord Hg and/or yearly measurements of equivalent hair Hg were available for 242 participants. Structural Equation Models (SEM) displayed the associations between Hg exposure and clusters, with Hg exposure modelled as a latent variable or in separate variables (prenatal, childhood and having had hair Hg ≥ 5 μg/g at least once over the sampling period). Longitudinal Mixed Effects Models (LMEM) served to examine past hair Hg with respect to clusters.
Results
A total of 37 symptoms bonded into 6 clusters, representing Extrapyramidal impairment, Sensory impairment, Cranial nerve disturbances, Gross motor impairment, Neuro-cognitive deficits and Affect/Mood disorders. Median Hg concentrations were 5 μg/L (1–78.5) and 1.1 μg/g (0.2–16) for umbilical cord and childhood hair, respectively. More than one-third (36.6%) had hair Hg ≥ 5 μg/g at least once. In SEM, latent Hg was directly associated with Extrapyramidal and Sensory impairment, Cranial nerve disturbances and Affect/Mood disorders. Direct associations were observed for prenatal exposure with Affect/Mood disorders, for childhood exposure with Extrapyramidal impairment and Cranial nerve disturbances, and for hair Hg ≥ 5 μg/g with Extrapyramidal and Sensory impairment. For all clusters, a further association between past Hg exposure and symptom clusters was mediated by diagnosed nervous system disorders. LMEM showed higher past hair Hg among those with higher scores for all clusters, except Affect/Mood disorders.
Conclusion
Our findings provide evidence that in this First Nation community, past Hg exposure from fish consumption was associated with later-life clusters of coexisting symptoms of nervous system dysfunction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-022-00838-y.
Keywords: Mercury, Past exposure, First nation, Nervous system dysfunction, Symptom clusters, Clustering, Structural equation modelling, Mixed effects model
Background
In the 1960’s, a chloralkali plant discharged approximately 10,000 kg of mercury (Hg) into the Wabigoon-English River system in Northwestern Ontario, contaminating fish resources as far as 250 km downstream [1]. Asubpeeschoseewagong Netum Anishinabek (Grassy Narrows First Nation) and Wabaseemoong Independent Nation (previously known as Whitedog and Islington Bands), for whom walleye (Sander vitreus) is central to their traditions, culture, economy and health, were seriously affected by this disaster [2]. Between 1970 and 1997, governmental agencies carried out biomonitoring programs in these communities to assess Hg concentrations in blood and hair [3]. Another program (1970–1992) assessed Hg concentrations in umbilical cord blood collected at the local hospital [3, 4]. Biomarker Hg concentrations from Grassy Narrows First Nation followed a similar pattern to Hg levels in local fish from the contaminated river system, with extremely high concentrations in the early 1970’s, a sharp decline until 1977 and a less pronounced decline until 1987, after which, mean concentrations remained relatively stable [5, 6].
In 1975, neurological examinations of 89 residents from the two communities, carried out by Dr. Masazumi Harada and colleagues [7], showed a high prevalence of signs and symptoms, similar to those reported by patients with Minamata Disease. The team revisited the communities in 2002 and 2004 and examined 175 individuals. Sixty (n=60) persons were diagnosed with Minamata Disease, 54 with Minamata Disease with complications due to other diseases, and 25 with “suspicion of Minamata Disease” [7]. Self-reported symptoms included numbness, pain in limbs, joints and back, decreased vision, impaired hearing, cramps in limbs, dizziness, tendency to fall, forgetfulness, impaired finger movement, tremor and speech impairment. For 27 persons who had been examined in 1975, symptoms had worsened [7]. Takaoka and co-authors [8] compared self-reported symptoms of volunteers from the Grassy Narrows First Nation community from examinations performed in 2010, with residents from the Minamata area and a reference group from other areas of Japan. The authors observed that the prevalence of specific and non-specific complaints of older persons from Grassy Narrows was similar to those from the Minamata area, while the prevalence among younger persons from Grassy Narrows was lower, but higher than the Japanese reference group [8]. While signs and symptoms reported in this study are consistent with methylmercury poisoning [9], the findings are limited by the recruitment strategy and the absence of measurements of mercury exposure.
In 2015, Grassy Narrows First Nation undertook a Community Health Assessment survey (GN-CHA), which included a list of 59 symptoms, as well as questions on current and past fish consumption. Although understanding one single symptom is worthwhile per se, people rarely present with one symptom, but rather with an array of multiple coexisting symptoms [10]. Clustering bonds concurrently occurring symptoms [10], whose pattern reflects the nature of the underlying dysfunction [11]. Multidimensional approaches and statistical techniques have been proposed to facilitate the integrated analysis of multiple symptoms [10, 12–15]. Also, a growing body of research has used advanced statistical techniques to examine the contributions of Hg exposure [16–26]. The objective of the present study was to bond GN-CHA reported symptoms of nervous system dysfunction into clusters and to examine their association to long-term and past Hg exposure.
Methods
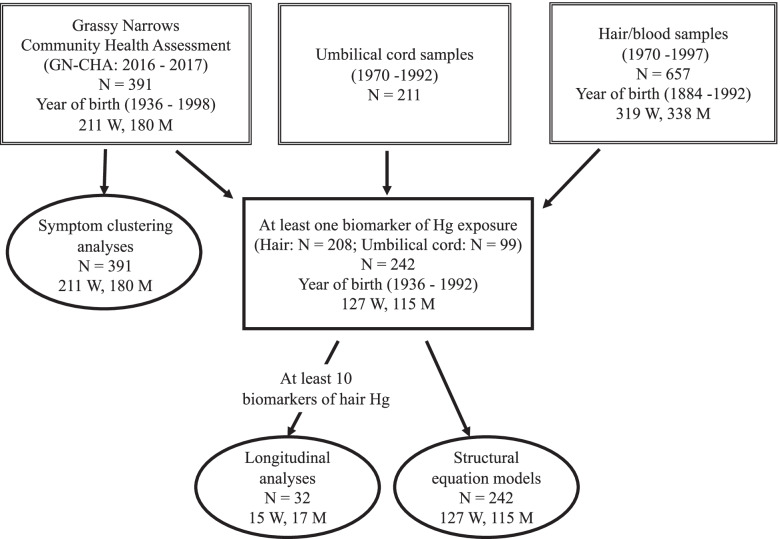
For the present study, carried out in collaboration with the Grassy Narrows First Nation community, we merged information from the GN-CHA survey and two government biomarker monitoring programs (umbilical cord Hg and blood and/or hair Hg) to examine the associations between current reported symptoms from the GN-CHA survey and past Hg exposure, using cluster analyses, structural equation models (SEM) and longitudinal mixed effects models (LMEM) (Fig. 1).
Fig. 1.
Flow chart of sample size in databases, selection criteria and analyses
GN-CHA survey
The GN-CHA survey for adults (18 years old and over), initiated in 2015, included 266 questions that covered many aspects of their life and health: demographics, education, generational attendance of residential school, work and income, food consumption, health status, diabetes care, wellness and mental health, injuries, disability, health care access, physical activity, smoking, drinking and drug use, community wellness and traditional culture. Most questions were taken from the First Nation Regional Health Survey 2008/2010 (FNRHS 2008/2010) [27], which provided a basis for comparison with other First Nation communities in Canada. Specific questions were added about fish consumption at different periods of one’s life, as well as illnesses and symptoms consistent with Hg poisoning. The questionnaire was pre-tested with 10 community members in July 2016. A house-to-house survey design was used, with the questionnaire on a web-based platform.
Survey administration was supervised by two field coordinators from Grassy Narrows; nine local interviewers went from house-to-house between December 2016 and March 2017. A total of 213 houses were identified on the reserve; 83.6% of houses were surveyed. Persons living off-reserve were recruited using convenience sampling. The results of the survey were presented to and discussed with community members during several small group and community meetings. The final report was approved by Chief and Council and a summary was made public on May 24, 2018.
A total of 391 Registered Grassy Narrows First Nation members (Band members) participated in the GN-CHA survey. At the time of the survey, 303 were living on-reserve and 88 off-reserve.
For the present study, we used the following variables from the GN-CHA: Demographics: age, sex, living on/off reserve, schooling, generational attendance of residential school in the family. Work: currently working (yes/no), currently looking for work (yes/no), reasons for not working (disability or illness/other). Food security: struggle to pay for food once a month or more in the last 6 months. Fish consumption: childhood fish consumption at 10 years of age (5 categories grouped into once a month or less/at least several times a month); walleye consumption over the past year (not at all/a few times/often). Lifestyle: current smoking (yes/no) and current alcohol consumption (heavy drinking (yes/no), defined in the FNRHS 2008/2010 [27] as 5 drinks in one drinking occasion at least once/month in the past 12 months). Health status: obesity (yes/no) was categorized using Body Mass Index ≥30 kg/m2 and at least one reported diagnosed chronic health conditions (yes/no), as listed in the FNRHS 2008/2010 (allergies, arthritis, asthma, cancer, chronic back pain, chronic bronchitis, diabetes, emphysema, heart disease, hepatitis, high blood pressure, liver disease, osteoporosis, rheumatism, stomach or intestinal problems, thyroid problems, and tuberculosis) and at least one diagnosed nervous system disorder (blindness, epilepsy, Bell’s palsy, cerebral palsy, muscular dystrophy, Kennedy’s disease, Parkinson’s diseases, Alzheimer’ disease, senile dementia, psychological/nervous disorders, cognitive/mental disorders, Attention deficit hyperactivity disorders (ADHD), learning disability).
The GN-CHA included 59 self-reported symptoms, rated on a 5-point Likert rating scale (“Never”, “Rarely”, “From time to time”, “Very often”, “All the time”). Higher scores on the Likert rating scale indicate greater frequency of symptoms.
Historic Hg biomarker data
At the request of Chief and Council of Grassy Narrows First Nation, the authors obtained from the First Nations and Inuit Health Branch (FNIHB) of the Ministry of Indigenous Services Canada and the Ontario Ministry of Health and Long-term Care, archived Band members’ historic biomarker data (hair and/or blood Hg concentrations), gathered between 1970 and 1997 and umbilical cord Hg data, collected between 1970 and 1992 at the hospital where Grassy Narrows women gave birth (Fig. 1).
Blood and hair samples were taken as part of a monitoring program of the Medical Research Branch of Health Canada [4]. The objective of the program was to identify persons whose Hg biomarker concentrations surpassed the guidelines of the time. There was no sampling strategy. The program primarily targeted fishing guides and their families, but anyone could volunteer and provide a sample [4]. Some years. The sampling period was longer than others and there was no consistency in the month that sampling was carried out.
Samples were analysed for total Hg at Health Canada laboratories [4, 28, 29]. According to Farant et al. 1981 [28], in the initial years, sample analyses were performed according to the Magos method [29] and later, a more efficient and less time-consuming method used an improved cold-vapor atomic absorption technique. The two methods were highly correlated for blood (r = 0.98) and hair (r = 0.97) [28].
Past Hg exposure databases
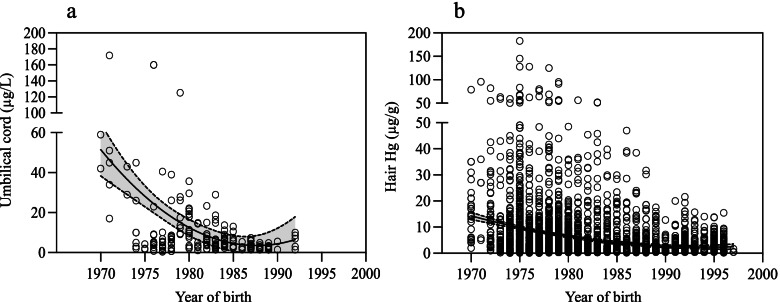
We created a past Hg exposure database, which included umbilical cord Hg concentrations for 211 newborns from Grassy Narrows, born between 1970 and 1992 (Fig. 2a) and blood/hair monitoring data, collected between 1970 and 1997, from persons born between 1884 and 1992. For the latter, we used the highest measure of equivalent hair Hg concentration for each year sampled; the details are described elsewhere [6]. A total of 3603 year-based equivalent hair measurements were available over the sampling period for 657 persons (Fig. 2b),
Fig. 2.
Distribution of Hg biomarker concentrations over time. a Umbilical cord blood Hg (μg/L) collected between 1970 and 1992 (n = 211). b Equivalent hair Hg (μg/g) from samples collected between 1970 and 1997 (n = 657)
For the present study, we selected GN-CHA participants with at least one biomarker measurement: either an umbilical cord Hg measurement (n = 99) and/or at least 1 year-based hair Hg concentration (n = 208; 1018 measurements) for a total of 242 persons. The number of year-based hair Hg measurements for each person varied between 1 and 23; 75 persons (36.1%) had 5 or more measurements, while 32 (15.4%) had 10 or more. For childhood Hg exposure, we used the mean hair Hg concentration between 5 and 15 years of age (n = 137 (56.6%)). Childhood Hg concentrations were significantly higher for persons who reported childhood fish consumption frequency at least several times a month compared to those who ate less (Additional file 1, Fig. 1). Moreover, those who ate fish at least several times a month and had attended a residential school (outside of the community) had lower Hair Hg at 10 years of age (3.8 μg/g [1.7–5.9] (n = 16) vs. 5.8 μg/g [4.2–7.3] (n = 30)).
Estimated past Hg exposures
For participants who did not have an umbilical cord Hg measurement, estimated Hg values were predicted from linear regression models using the 211 umbilical cord data (Fig. 2a) on the year of birth for the three periods of Hg exposure (1970–1976, 1977–1987 and 1988–1992). These estimates were then adjusted on where the person’s mother had spent her pregnancy and/or gave birth. For those who were born or whose mother spent her pregnancy in the Wabigoon-English River region between 1970 and 1992, the mean measured umbilical cord blood Hg for their year of birth was used. For those born prior to the Hg discharge in 1962, we attributed 1.0 μg/L for cord blood Hg, while for those born between 1962 and 1970, mean cord blood Hg in 1970 was used (65.7 μg/L), and for those born after 1992, mean cord blood Hg from 1992 was applied (4.48 μg/L). Finally, for individuals, whose mother spent her pregnancy and delivered elsewhere, cord blood Hg was set at 1.0 μg/L.
For study participants, who did not have childhood hair Hg values, we derived estimates from the yearly-hair Hg database (n = 657), using the overall mean hair Hg for the year in which they turned 10 years of age. For participants, who were ≥ 10 years old in 1962 (beginning of the discharge) childhood hair Hg was set at 0.1 μg/g. For those who were 10 years of age between 1963 and 1970, the mean measured hair Hg in children in 1970 was used. Since there were few Hg data after 1990 and there appears to be a plateau over this last period (Additional file 1, Fig. 1), we attributed 0.81 μg/g for those who were 10 years of age after 1990. For all those who were 10 years of age after 1962, estimates were then adjusted on reported childhood fish consumption and having attended a residential school. We were unable to estimate childhood hair Hg exposure for 13 persons (5.4%) of the 242 study participants, who were missing hair Hg data between 5 and 15 years old and who did not answer the question about childhood fish consumption in the GN-CHA survey.
To validate the estimated Hg measures, we tested the correlations between measured and estimated values for both umbilical cord and childhood hair Hg, using non-parametric tests (Spearman).
Symptom clustering
Self-reported symptoms from the GN-CHA survey (n = 391) were clustered based on their Likert scale, using a simultaneous two-step multivariate segmentation approach [30–32]. One is agglomerative and aims at maximising a homogeneity criterion by successively aggregating the variables into clusters using a hierarchical ascendant clustering algorithm. The other is representative and creates a composite variable from a weighted linear combination of symptoms (synthetic variable that can be read as a gradient) for each cluster using a mixed factorial approach (PCAMIX method) [30, 32, 33]. For each composite variable (cluster), all participants received a score based on his/her symptom frequency.
A bootstrap approach was used for maximizing the homogeneity criterion within clusters and determining the suitable number of clusters. The desired number of clusters (K) was determined from the analysis of aggregation levels, stability of the partitions via bootstrapped mean-adjusted Rand Index and boxplots. The mean-adjusted Rand index was based on the generation of 60 bootstrap samples. Each cluster was carefully inspected based on its proportion of variation explained (a minimum of 50% was mandatory) and on degree of “closeness” among the symptoms being clustered (a minimum of 0.6 squared correlation of the variable with its composite variable was required). Some symptoms add noise while providing little or no information in identifying the underlying pattern inherent to the cluster [34]. Finally, we tested for the “driving” symptoms that best reflect the cluster. We validated the cluster results, using the VARCLUS function of the SAS computer application (JMP Professional 15.0 software).
A confirmatory factor analysis (CFA) was conducted to evaluate the construct validity of symptom clustering using a series of fit parameters [35–39], using the following indices: Chi-square divided by the degrees of freedom (χ2/df), Standardized Root Mean Square Residual (SRMR), Root Mean Square Error of Approximation (RMSEA), Comparative Fit Index (CFI) and Tucker Lewis Index (TLI). Internal consistency (scale reliability) for each cluster was tested using the Cronbach alpha [35, 40–42]. Construct validity for each cluster was also confirmed by the neurologist (T. S. Lena).
Structural equation models
SEM is a comprehensive methodology for representing and simultaneously testing a network of complex relationships. This technique can incorporate observed (measured) and unobserved variables (latent), while traditional techniques only allow for measured variables. The latent variable is an unobservable multidimensional construct that cannot be directly measured [43, 44]. SEM allows for data from multiple exposures and multiple outcomes to be considered simultaneously [45]. It is based on an interrelated system of linear regression equations that extend the possibility of relations between observed and latent variables, as well as direct and indirect (mediation) relations and allows for flexibility in modeling covariance structures [19, 39, 46, 47]. Finally, SEM provides path diagrams that are useful to understand and interpret the contribution of the various variables. The standardized factor loadings allow for pathway comparison.
In the SEM, we used the latent variable for symptom clusters rather than the composite variable because it better reflects the structural concept of co-occurring symptoms. A latent variable was likewise used for Hg exposure at different times (prenatal, childhood and having had ≥5 μg/g hair Hg at least once over the monitoring period). Each cluster was analysed using two SEM, the first with the latent Hg exposure variable and the second with the Hg exposure variables at the three different times. We tested possible direct and indirect (mediation) associations between Hg exposure variables and the symptom clusters. Age, sex, year of sampling, residence, as well a series of variables representing socioeconomic status (income, struggle to pay for food), drinking, smoking, obesity and medical history (diabetes, hypertension, heart disease, stroke, hypercholesteremia, separately and/or grouped into the variable “at least one diagnosed chronic health condition”), were tested a priori as covariates. We further tested whether sex modified the relation between past Hg exposures and symptom clusters (moderation). Moderated mediation was also tested with age and sex.
Variables with a skewed distribution were log (base 10) transformed. Because latent variables, Hg exposures and covariates, are all on different scales, we used standardized path coefficients to test the magnitude of relations. Standardized coefficients indicate the expected amount of change in standard deviation (SD) in the dependent latent symptoms cluster for an increase of one SD in the predictor, while other predictors (covariates) are kept constant. The adequacy of model fit to the data was determined using multiple indices: χ2/df, CFI, TLI, RMSEA, SRMR and Akaike’s Information Criterion (AIC) and Schwarz’s Bayesian information criterion (BIC) [35–39]. Diagonally weighted least squares (DWLS) adjustments in SEM were used to take into account the ordinal nature of symptom frequency outcomes. To improve the reliability of the latent construct’s scale or the models, we examined residual variances between indicators. Modification indices (MI) were used to assess the addition or deletion of variables and/or associations to improve the goodness of fit of the SEM. The sequence of initial fit, MI and refitting were repeated until the best fit SEM was attained.
To ensure that the database of 242 participants was sufficient to run SEM, two types of power analyses were performed: i) to detect model misspecification and ii) to detect target effect (influence of Hg exposure on the symptom cluster) [48]. A-priori, post-hoc, and compromise power-analyses were run simultaneously to detect model misspecification based on Chi-square likelihood-ratio and RMSEA tests of close and not-close fit, [49–52]. The power for detecting specific target effect was determined with simulated data in SEM [48].
For the SEM, validated prenatal and childhood Hg exposure estimates, described above, were used when there was no measurement, while for GN-CHA variables, missing data were imputed using Multivariate Imputation by Chained Equations (MICE).
To support the SEM, we ran a series of Directed Acyclic Graphs (DAG) for sensitivity analyses. DAG is a visual representation of causal assumptions that makes underlying relations explicit. It is used to detect overt bias, notably through backdoor paths that identify the presence of confounding [53, 54]. Modifications to DAG-implied adjustment sets include conditioning confounding and variables with ambiguous causing roles [55, 56].
Longitudinal mixed effects models
LMEMs were performed using direct measurements of longitudinal hair Hg with respect to the composite variable for each cluster. We chose mixed effects modeling (MEM) as it is robust to missing data and irregularly spaced sampling; it also handles both time-invariant and time-varying covariates [57]. MEM enables multilevel modeling and partitioning of the covariance structure (random effects). We tested a priori the following covariates (age, sex, variables of lifestyle and medical history, age at sampling and sampling season). The normality of residuals was tested using a q-q plot. The most appropriate model was selected using the Akaike Information Criterion (AIC), the Baysian Information Criteria (BIC) and the likelihood ratio (LR) test at p < = 0.05.
For the longitudinal approach, LMEM requires repeated measures. Because of the absence of a biomarker sampling strategy, over half (56%) of the 208 persons with hair Hg measurements had 3 repeated measurements or less. Despite the flexibility of MEM with missing data, we preferred not to impute Hg levels for people whose diet and environmental context varied over time, particularly when living off-reserve. For LMEM, we only included persons with at least 10 hair Hg measurements (n = 32 (17 men and 15 women)), with a total of 447 hair Hg measurements, covering a period from 10 to 23 years (median 21 years).
To ensure that there were sufficient observations for the analyses, we estimated the minimal required sample size, based on formulas from Hedeker and co-authors [58] and direct calculations using the G*power software [59–61]. Since one centimeter of a hair sample represents an accumulation of Hg during approximately 1 month [62], we used a low correlation of repeated measures between yearly-based samples (rho 0.1 and 0.2). Because the effect size was unknown, 0.25 was chosen. Power analyses were set at 80%, with a two-tailed 5% hypothesis test, 10 time points and up to four fixed factors; the minimum number of participants required was between 29 and 42.
Threshold of statistical significance in all analyses was set at p ≤ 0.05.
Database management and descriptive statistical analyses were performed using JMP Professional 15.0 (Statistical Analysis Hardware (SAS Institute). LMEM were conducted with Stata 16 software (StataCorp. 2019. Stata Statistical Software: Release 16.0. College Station, TX: Stata Corporation). All other analyses were computed using the R statistical software version 3.6.1. (R Core Team, 2016). We used the R package ClustOfVar [31] for clustering analyses and the MICE (Multiple Imputation by Chained Equations) package for multiple imputation of missing data in CFA and SEM. PairedData and dplyr packages were used for paired-t tests. CFA and SEM models were computed with the R package Lavaan [39]. The dagitty and ggdag R packages were used for analysing SEM and DAG. A-priori, post-hoc, and compromise power-analyses were computed using the semPower package. To assess power for detecting a target effect in SEM we used an online tool: yilinandrewang.shinyapps.io/pwrSEM/ [63].
Results
Relevant characteristics of the 391 participants in the GN-CHA survey, whose data were used for symptom clustering, are presented by age category in Tables 1 and 2. In this community, age also reflects changes in Hg exposure over time. Almost half of the participants were between 30 and 49 years of age, with no difference in age distribution between women and men. There were important age differences in schooling. Proportionally fewer older persons had attended high school, however, over half of those 30 years and older had furthered their education through post-secondary training programs (data not shown). In Canada, First Nation children were placed, often forcibly, in residential schools, with harmful effects on themselves and their families [64, 65]. In the present study, no one born after 1978 had attended residential school; for those born prior to this date, 58 persons (50.0%) had been in a residential school, while for their mothers and/or fathers, it was 95.4%. At the time of the survey, 51.1% of women and 43.1% of men were working, excluding persons studying, retired or at home with children (n = 32). Among participants who reported not working, 78% of those between 18 and 49 years of age were looking for work, while 31 persons (68.9%) of those over 30 years of age, who were not looking for work, reported that it was due to illness or disability.
Table 1.
The relative frequency of the characteristics of the population within each age category
| Na | 18–29 y n (%) |
30–49 y n (%) |
50+ y n (%) |
Total n (%) |
Chi-square (LR)b p-value |
|
|---|---|---|---|---|---|---|
| Sex | 391 | |||||
| Women | 66 (53.3%) | 97 (51.6%) | 48 (60.8%) | 211 (54%) |
1.94 0.380 |
|
| Men | 58 (46.7%) | 72 (48.4%) | 31 (39.2%) | 180(46%) | ||
| Living on reserve | 390 | 95 (76.6%) | 144 (77.0%) | 62 (78.5%) | 301 (77.2%) |
0.10 0.949 |
| Schooling | ||||||
| Attended school | 382 | 122 (100%) | 180 (98.4%) | 75 (97.4%) | 377 (98.7%) |
4.13 0.257 |
| Attended high school | 366 | 115 (98.3%) | 166 (94.9%) | 48 (64.9%) | 329 (89.9%) |
52.6 < 0.0001 |
| Other education | 386 | 33 (27.1%) | 108 (58.4%) | 43 (54.4%) | 184 (47.7%) |
31.69 < 0.0001 |
| Residential school | ||||||
| Attended residential school | 376 | 0 | 6 (3.4%) | 52 (66.7%) | 58 (15.4%) |
171 < 0.0001 |
| Parents in residential school | 326 | 36 (40.5%) | 148 (89.7%) | 68 (94.4%) | 252 (77.3%) |
89 < 0.0001 |
| Work activities | ||||||
| Currently workingc | 349 | 39 (37.5%) | 94 (52.2%) | 32 (48.5%) | 165 (49.2%) |
5.90 0.052 |
| Currently looking for workc | 165 | 45 (81.8%) | 58 (75.3%) | 7 (21.2%) | 110 (66.7%) |
38.2 < 0.0001 |
| Struggle to pay for food monthly or more in the last year | 332 | 28 (26.9%) | 38 (24.2%) | 17 (23.9%) | 83 (25.0%) |
3.65 0.392 |
| Current smoker | 376 | 54 (46.2%) | 90 (49.7%) | 24 (30.8%) | 168 (44.7%) |
0.28 0.016 |
| Heavy drinker | 360 | 79 (71.2%) | 100 (56.8%) | 20 (27.4%) | 199 (55.3%) |
35.3 < 0.0001 |
| Obese | 375 | 47 (39.8%) | 93 (51.7%) | 36 (46.8%) | 176 (46.9%) |
4.03 0.133 |
| At least one diagnosed chronic health condition | 385 | 46 (38.3%) | 117 (62.9%) | 67 (84.8%) | 230 (59.7%) |
46.6 < 0.0001 |
| At least one diagnosed nervous system disorder | 381 | 20 (17.1%) | 37 (19.9%) | 35 (44.9%) | 92 (24.2%) |
32.6 < 0.0001 |
aNumber (N) of valid responses (does not include don’t know or don’t remember and refused)
bLikelihood Ratio Chi-square compares the relative frequency between age categories
cExcludes students, stay at home parents and retirees
Table 2.
The relative frequency of fish consumption by age category
| N | 18-29 y | 30-49 y | 50+ y | Total | Chi-square | |
|---|---|---|---|---|---|---|
| n | n | n | n | (LR) | ||
| (%) | (%) | (%) | (%) | p-value | ||
| Fish consumption at 10 y of age | 333 | |||||
| None | 9 (9.4%) | 10 (6.1%) | 4 (5.5%) | 23 (6.9%) |
101 <0.0001 |
|
| Hardly ever/occasionally | 32 (33.3%) | 26 (15.9%) | 3 (4.1%) | 61 (18.3%) | ||
| Once a month | 24 (25.0%) | 38 (23.2%) | 2 (2.7%) | 64 (19.2%) | ||
| Several times a month | 28 (29.2%) | 96 (42.1%) | 24 (32.9%) | 121 (36.4%) | ||
| Every day | 3 (3.1%) | 21 (12.8%) | 40 (54.8%) | 64 (19.2%) | ||
| Walleye consumption over past year | 382 | |||||
| Not at all | 23 (19.5%) | 29 (15.7%) | 9 (11.4%) | 61 (16.0%) |
3.44 0.486 |
|
| A few times | 60 (50.9%) | 96 (51.9%) | 48 (60.8%) | 204 (53.4%) | ||
| Often | 35 (29.7%) | 60 (32.4%) | 22 (27.9%) | 117 (30.6%) |
Likelihood Ratio Chi-square compares the relative frequency between age categories
There was no age difference for difficulty paying for food, which was reported by almost 25% of participants. The prevalence of current smokers and heavy drinkers significantly decreased with age. Obesity was most prevalent among those 30–49 years old, of whom two-thirds were women. Sixty percent of participants reported that a health professional had told them that they had at least one chronic health condition; the prevalence increased significantly with age.
There were no differences between men and women for age distribution, schooling, having attended or having parents who attended residential school, difficulty paying for food, and current smoking. Proportionally more men reported heavy drinking compared to women (62.7% vs 48.7%; Chi-square LR 7.17; p = 0.007), while women had a significantly higher prevalence of obesity (54.0% vs 38.9%; Chi-square LR: 14.01, p = 0.003) and at least one chronic health condition diagnosed by a health professional (68.5% vs 49.7%; Chi-square LR: 14.01, p = 0.0001).
In this community, fish, especially walleye, is the source of Hg exposure [4, 5]. Table 2 contains the responses to the GN-CHA questions on past and current fish consumption frequency. Over half of those 50 years and older (born before 1968) reported that, as a child, they ate fish daily. Childhood fish consumption frequency significantly decreased over time. Fish consumption questions were species specific. Walleye is still the fish the most consumed; 30.6% of persons surveyed in the GN-CHA reported often eating walleye compared to 4.7% for whitefish and 3.2% for northern pike. No differences were observed between women and men for the frequency of childhood or current fish consumption.
For symptom clustering of the GN-CHA, aggregation levels and construct stability are presented in Additional file 1, Fig. 2. Aggregation distances supported a cutting threshold between 5 and 8 clusters (Additional file 1, Fig. 2a), while the bootstrapped mean-adjusted R and criterion suggest 6, 7, 11, or 12 (Additional file 1, Fig. 2b). The boxplots (Additional file 1, Fig. 2c) showed that the highest stability of the partition solution varies between K5 and K9, with K7 showing a slightly higher median value, but a lower dispersion around the mean. After verification of all the above criteria, we kept a total of six clusters. Goodness of fit for CFA was acceptable for the six clusters (CFI = 0.85, TLI = 0.84, RMSEA = 0.08, SRMR = 0.05), with a narrow 90% CI, representing a high degree of precision. The Cronbach alpha (> 0.8) for each cluster confirmed internal consistency (Additional file 1, Supplementary Table 1).
The six clusters included a total of 37 symptoms. Additional file 1, Supplementary Table 1 presents the list of symptoms retained in each cluster with its correlation. Cluster 1 (Extrapyramidal impairment), which aggregated the highest number of symptoms (n = 9), included tremors, balance impairment, functional limb weakness and pain in arms and legs, reflecting an extension of extrapyramidal neurological symptoms. Cluster 2 (Sensory impairment) included peripheral neuropathic deficits, notably upper and lower limb sensory impairments including numbness, dullness and tingling. Cluster 3 (Cranial nerve disturbances) included anosmia, ageusia, dysphagia and tingling around the mouth. Cluster 4 (Gross motor impairment) aggregated symptoms that affect carrying, lifting and walking. Cluster 5 (Neuro-cognitive deficits) groups cognitive dysfunction associated with memory loss, hearing impairment and speech disorders. Finally, Cluster 6 (Affect/Mood disorders) included anxiety, irritability, depression and sleeping issues. For each cluster, there was no particular driving symptom. Most symptoms were highly related to their own cluster (Additional file 1, Supplementary Table 1).
For the large majority of symptoms, no significant difference was observed in the proportion of men and women who reported its frequency as very often/all the time (Additional file 1, Supplementary Table 1). Symptom frequency increased significantly with age (data not shown), with the exception of difficulty pronouncing words, forgetting to do things, doing nothing, irritability, anxiety, difficulty falling asleep, waking up at night, depressed, tiredness and difficulty concentrating (Fisher’s Exact Test; p ≥ 0.08).
The descriptive characteristics of the composite variable for each cluster for women and men are presented in Additional file 1, Table 2. A higher score in the composite variable reflects a higher frequency of coexisting symptoms. Scores were significantly higher in women than in men, with the exception of Neuro-cognitive deficits. Scores increased significantly with age (Rho: 0.138–0.409; p < 0.01), with the exception of Affect/Mood disorders (Rho: 0.108; p = 0.095).
Of the original GN-CHA participants (n = 391) who were used to create the clusters, 242 were selected on the basis of having at least one Hg biomarker measurement (Fig. 1). Umbilical cord, childhood hair Hg concentrations and the percentage of persons with at least one hair mercury concentration ≥ 5 μg/g with respect to age category are presented in Table 3. There was a positive association between childhood fish consumption and measured hair Hg, adjusted for sex and year of sampling (MEM: Wald Chi-square = 96.9; p = 0.003). Paired t-tests, comparing mean hair Hg at 10 years of age, for each year, between those who reported eating fish during childhood at least several times a month and those who reported eating less, supported the MEM result (Wilcoxon Signed-Rank prob.>|S|, S: 37.5; p = 0.020).
Table 3.
Measured mercury concentrations by age category
| N | 18–29 y Median (min-max) |
30–49 y Median (min-max) |
50+ y Median (min-max) |
Total Median (min-max) |
Wilcoxon test p-value |
|
|---|---|---|---|---|---|---|
| Umbilical cord (ppb) | 99 |
3.5 (1.5–10) |
5.6 (1–78.5) |
– |
5 (1–78.5) |
0.023 |
| Childhood Hair Hg (μg/g) | 137 |
0.9 (0.5–2) |
0.7 (0.2–16) |
2.9 (0.6–15) |
1.1 (0.2–16) |
< 0.0001 |
| n (%) | n (%) | n (%) | ||||
|
Hair ≥ Hg 5 μg/g at least once |
208 | 0 |
27 (22.9%) |
49 (74.2%) |
76 (36.6%) |
< 0.0001 |
Persons retained for the SEM analyses (n = 242) had similar characteristics to the original participants (Tables 1 and 2), although they were slightly older (median: 39 years old; interquartile range: 32–50), with proportionally fewer persons in the 18–29 y old category (13.6%). Compared to the entire cohort (Table 2), more young persons reported eating fish frequently during their childhood (41.6%), and often consuming walleye during the past 12 months (41.9%).
In the SEMs, measured and estimated Hg values were used when measures were missing. Validation of estimated Hg values showed significant associations with direct measurements for both prenatal and childhood Hg exposure (n = 99; Rho = 0.22; p = 0.023 and n = 137; Rho = 0.73; p < 0.0001, respectively). Most participants (n = 197 (81.4%)) were born after 1962 (when exposure began) and had both pre- and post-natal exposures. Only 8 of the 242 persons (3.3%) were over 15 years of age in 1962.
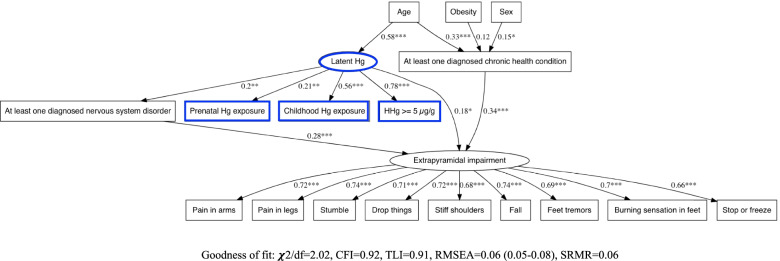
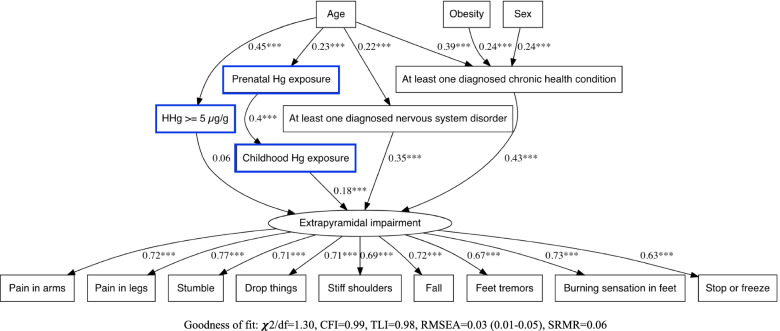
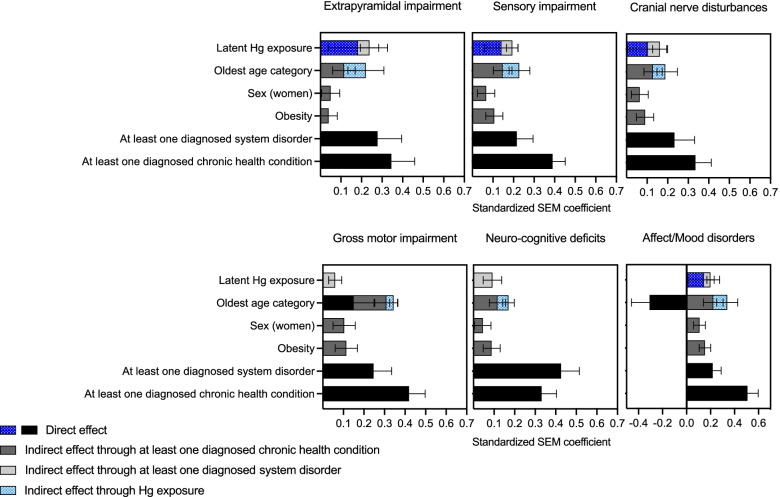
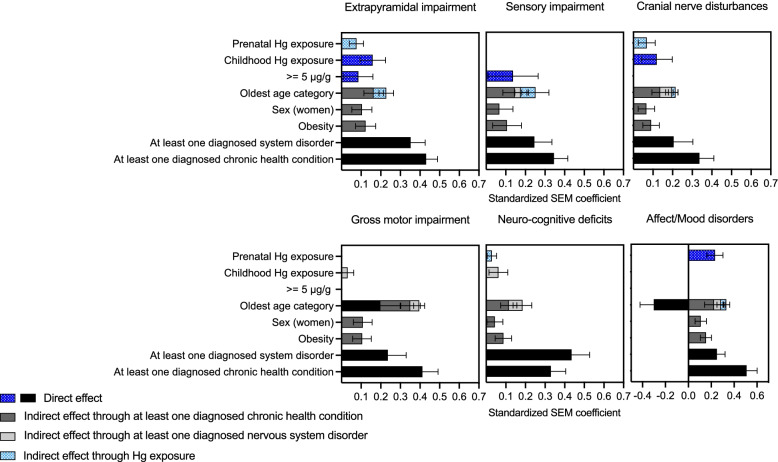
For each symptom cluster, SEMs were performed (i) with latent Hg and (ii) with prenatal, childhood and hair Hg ≥ 5 μg/g. Path diagrams, showing the configuration of the different pathways and the one-to-one relation between variables, are illustrated in Figs. 3 and 4 and in Supplementary Figs. 3–12 in Additional file 1. SEM standardized path coefficients, representing the total quantified contribution of significant direct and indirect associations, are presented in Figs. 5 and 6.
Fig. 3.
SEM path diagram linking retrospective latent Hg exposure and covariates to the latent symptoms cluster variable for Extrapyramidal impairment. Abbreviations: χ2/df: Chi-square divided by the degrees of freedom (χ2/df), CFI: Comparative Fit Index, TLI: Tucker Lewis Index, RMSEA: Root Mean Square Error of Approximation, SRMR: Standardized Root Mean Square Residual
Fig. 4.
SEM path diagram linking retrospective Hg exposure parameters (prenatal and childhood Hg exposure) and having had ≥5 μg/g hair Hg at least once between 1970 and 1997) and covariates to the latent symptoms cluster variable for Extrapyramidal impairment. Abbreviations: χ2/df: Chi-square divided by the degrees of freedom (χ2/df), CFI: Comparative Fit Index, TLI: Tucker Lewis Index, RMSEA: Root Mean Square Error of Approximation, SRMR: Standardized Root Mean Square Residual
Fig. 5.
SEM standardized path coefficients for direct and indirect contributions of latent Hg and covariates on the symptom latent variable for each cluster
Fig. 6.
SEM standardized path coefficients for direct and indirect contributions of prenatal, childhood and ≥ 5 μg/g hair Hg (1970–1997) and covariates on the symptom latent variable for each cluster
For all latent Hg-based SEMs, childhood Hg exposure and hair Hg 5 μg/g were the main drivers in the construct of latent Hg (Figs. 3 and 5). Latent Hg showed a direct contribution to most symptom clusters, but not Neuro-cognitive deficits and Gross motor impairment. For all clusters, there was also a pathway mediated through having at least one diagnosed nervous system disorder.
In every SEM with Hg exposure variables for prenatal, childhood and hair Hg ≥ 5 μg/g, there was a significant relation between prenatal and childhood exposure (Figs. 4 and 6 and Supplementary Figs. 8–12, Additional file 1). For Extrapyramidal impairment (Fig. 4) and Cranial nerve disturbances (Supplementary Fig. 9, Additional file 1), standardized path coefficients showed that the contribution of prenatal exposure to the cluster was mediated by childhood exposure.
For Sensory Impairment (Supplementary Fig. 8, Additional file 1), Gross motor impairment (Supplementary Fig. 10, Additional file 1) and Neuro-cognitive deficits (Supplementary Fig. 11, Additional file 1), there was a chain of mediation from prenatal exposure to childhood exposure and from childhood exposure to being diagnosed with at least one nervous system disorder. When the same chain of mediation was modelled for Extrapyramidal impairment and for Cranial nerve disturbances, the models’ fit parameters were weaker. In these cases, the direct association of childhood Hg with the symptom cluster was stronger than its indirect association through diagnosed nervous disorder.
Prenatal Hg exposure was directly associated with Affect/Mood disorders (Supplementary Fig. 12, Additional file 1). Hg ≥ 5 μg/g directly influenced Extrapyramidal and Sensory impairment (Fig. 4 and Supplementary Fig. 8 in Additional file 1, respectively).
The best fit models were those that included childhood exposure as mediator for prenatal exposure. Childhood exposure was not a confounding variable in the relation between prenatal exposure, diagnosed nervous system disorder or any symptom cluster. Moreover, there was no moderated mediation with age category or Hg exposure in the SEM.
Although of different magnitudes, age category, sex and obesity, at least one diagnosed chronic health condition and at least one diagnosed nervous system disorder, were directly and/or indirectly associated with symptom clusters in all SEMs. Positive associations were observed with age category with respect to diagnosed nervous system and chronic health conditions, with the exception of Affect/Mood disorders, for which age was both negatively (direct) and positively (indirect) associated with symptom frequency reporting (Supplementary Fig. 12, Additional file 1). Older persons tended to report fewer symptoms of Affect/Mood disorders (direct), unless they suffered from chronic health conditions and/or nervous system disorders (indirect). Past Hg exposure mediated the contribution of age for all clusters, except for Gross motor impairment (Supplementary Fig. 10, Additional file 1) and Neuro-cognitive deficits (Supplementary Fig. 11, Additional file 1).
Longitudinal MEM analyses were performed with all persons who had at least 10 year-based hair measurements (n = 32; 17 men and 15 women). Their median age was 54 years (interquartile range: 48–64). The associations between longitudinal hair Hg and the median of the composite variable for each cluster are shown in Table 4. Age, sex and year of sampling were included as fixed factors, and age at time of sampling as a random factor. In these analyses, 5 of the 6 clusters were significantly associated (p ≤ 0.05) or show a tendency (< 0.10) with longitudinal hair Hg. No association was observed between longitudinal hair Hg and Affect/Mood disorders.
Table 4.
Longitudinal Mixed Effects Model results for estimates of hair Hg concentrations from 1970 to 1997 with respect to the median of the cluster score
| Composite cluster variable a, b | Hair Hg Estimatec | % Confidence Interval | p -value |
|---|---|---|---|
| Cluster1 (Extrapyramidal impairment) | 1.76 | 0.0–3.6 | 0.061 |
| Cluster2 (Sensory impairment) | 1.57 | −2.7 - 3.4 | 0.096 |
| Cluster3 (Cranial nerve disturbances) | 2.60 | 1.0–4.2 | 0.001 |
| Cluster4 (Gross motor impairment) | 2.70 | 0.4–5.1 | 0.023 |
| Cluster5 (Neuro-cognitive deficits) | 2.41 | 0.6–4.2 | 0.009 |
| Cluster6 (Affect/Mood disorders) | −0.33 | −0.3 - 1.0 | 0.758 |
aOnly participants with 10 measurements and more of hair Hg are included in the analyses (32 persons; 447 Hair Hg measurements)
bSignificant covariates include age, sex, year of sampling (random effect: age of sampling nested in year of sampling)
cHair Hg estimates represent the difference in hair Hg with respect to cluster scores above and below the median over the sampling period
Discussion
To our knowledge, this is the first study to link past Hg exposure from freshwater fish consumption with clusters of current multiple coexisting symptoms. Clustering analysis enabled us to characterize the various symptom profiles, which constitute the physical and/or mental translation of structural phenomena. The non-random distribution of symptoms and their strong correlation in each cluster suggests a common mechanism or etiology [10, 12, 66]. Here, the six clusters, derived from statistical empirical validation, reflect the involvement of different aspects of nervous system dysfunction, which we classified as representing primarily Extrapyramidal impairment, Sensory impairment, Cranial nerve disturbances, Gross motor impairment, Neuro-cognitive deficits, and Affect/Mood disorders. The symptoms that make up these clusters were similar to those reported by persons from Grassy Narrows in 2010 [8, 67] and consistent with the many descriptions of methyl Hg poisoning through fish consumption [68–75].
SEM provided the opportunity to simultaneously examine covariance, mediation and moderation between variables through a domino effect of successive pathways from past exposure to current symptoms. The Hg exposure latent variable, which grouped different periods of exposure (prenatal Hg, childhood Hg and hair Hg ≥ 5 μg/g at least once), enabled us to examine their combined contributions. Latent Hg exposure variables have been used in other studies. Choi et al. [23] showed that a grouped Hg exposure variable, including current Hg nail and blood concentrations and hair Hg concentration 7 years previously, was associated with adverse cardiovascular outcomes in Faroese whaling men. In a study by Grandjean and co-authors [76], the combined prenatal and childhood Hg exposures were negatively related to children’s neurobehavioral outcomes. In the present study, the combined past Hg exposures were, directly and indirectly, associated with all clusters.
Hg biomarker data, collected in Grassy Narrows First Nation between 1970 and 1997 provided a unique opportunity to examine symptom clusters with respect to past exposure over a long-term period and at various time points. The findings of longitudinal analyses, based on participants with at least 10 year-based hair Hg measurements over the sampling period, showed that long-term Hg exposure was associated with higher symptom frequency for all the clusters of nervous system dysfunction, with the exception of Affect/Mood disorders. This is consistent with SEM findings where Affect/Mood disorders showed a significant relation only with prenatal exposure.
Independent Hg exposures may provide insight into the relative importance of exposure at different time periods. While prenatal exposure was directly associated with Affect/Mood disorders, for other clusters, its contribution was mediated by childhood exposure. Fetal Minamata patients present with psychiatric disorders (Harada, 1964 cited in Yorifuji et al. [77]). A re-analysis of 1971 data from Minamata patients showed that psychiatric symptoms peaked at 20 years of age [77]. This may be similar to the present study where young adults (≤ 29 years of age) reported a higher frequency of altered mood symptoms. The importance of prenatal and childhood Hg exposure has been raised by many authors [25, 78]. Children are more vulnerable to toxic exposure than adults because their brains are in a state of rapid growth, with relatively higher absorption rates to body weight; their body systems are not prepared to properly metabolize, detoxify, and excrete toxic substances [79–81].
Although the GN-CHA did not include the age of onset of symptoms, the association between past exposure and later-life symptoms raises the issue of delayed and/or progressive neurotoxicity. Delayed neurotoxicity was reported in 13-year-old monkeys, treated with methyl Hg from birth to 7 years of age [82]. A silent latency period for methyl Hg toxicity has been proposed using examples from the Minamata and Iraq disasters [83]. Newland and co-authors [84] discuss mechanisms by which neuronal development may have long-lasting behavioral consequences that appear in adulthood and, in some cases, may not appear until aging.
The FNRHS 2008/2010 included few nervous system disorders in their list of diagnosed health conditions [27]. The GN-CHA added a series of neurologic disorders which share some of the signs and symptoms of Hg poisoning. These disorders mediated the relation between latent Hg exposure and all symptom clusters and between childhood exposure and Sensory impairment, Gross motor impairment and Neuro-cognitive deficits.
In Canada, chronic health conditions are disproportionately higher among Indigenous communities compared to the non-Indigenous population [85]. The most commonly reported chronic health conditions in the GN-CHA are similar to other First Nation communities across Canada: high blood pressure, allergies, arthritis, diabetes, and chronic back pain [27]. Results of the SEM showed the expected associations of having at least one chronic health condition with age and obesity, with women presenting a higher prevalence compared to men. It is noteworthy that the highest direct contribution to all symptom clusters was having at least one diagnosed chronic health condition and/or nervous system disorder.
SEM remains one of the best approaches to assess simultaneously direct and indirect associations by investigating all relevant regression pathways. Our findings are consistent with follow-up studies of persons that had been affected by Hg pollution from the Chisso Company in Minamata, decades after the company had halted the dumping of methyl Hg into the bay [74, 86–88]. The Japanese studies compared persons who had lived in polluted and non-polluted regions since they did not have actual measures of Hg exposure for the individuals who were evaluated.
Some limitations need to be considered in interpreting the present results. The study reposes on self-reported questionnaire data from the GN-CHA, raising the question of recall bias, and under- or over-reporting. To offset these limitations, the Cronbach alpha and CFA confirmed the construct reliability of the clustered symptoms. Reported diagnosed health conditions and nervous system disorders reflected whether a person had consulted a health professional who had diagnosed this condition, as well as the accuracy of the diagnosis. Further studies would benefit from clinical assessments in relation to past Hg exposure.
A further limitation is with respect to Hg biomarkers obtained from a government monitoring program [3]. Sampling was not carried out regularly and samples were not collected yearly from everyone, resulting in many missing values. Moreover, there were seasonal and yearly differences in fish consumption frequency [3, 6]. Hg exposure imprecision could also be associated with blood and hair measurements over the sampling years, notably laboratory measurement imprecision and biological variation [89]. Ideally, one would like to have had continuous Hg biomarker measurements over all participants’ lifetime, but this was not possible. To address the challenges posed by the biomarker database, for the SEMs, the estimated umbilical cord and childhood Hg values were validated, and we used latent variables of Hg exposure to manage residual error terms between variables. For the longitudinal MEMs, we limited the analyses to persons with a minimum of 10 hair Hg measurements, representing exposure for 10 years or more.
Studies on Minamata Disease have shown differences in fetal, non-fetal infantile and adult Hg damage to the brain [90]. In the present study, the large majority of persons experienced both prenatal and postnatal exposure. The mediated contribution of prenatal exposure on symptom clusters through childhood exposure, observed in most SEMs, suggests that their independent contributions are difficult to assess. While both prenatal and childhood Hg exposures decreased over time, reflecting diminishing fish Hg concentration and changing family dietary patterns, they also represent different stages of neurodevelopment and have potentially different effects. Our data showed that childhood Hg exposure may be a mediator/cumulative effect of prenatal exposure, except for Affect/Mood disorders. We tested childhood exposure alone, but the associations were stronger when prenatal exposure was included in the model.
The Grassy Narrows Hg disaster began in 1962 and after 1970, fish Hg concentration and fish consumption decreased over time, paralleled by a decrease in biomarker Hg concentrations. Associations with Hg exposure, observed in the models, may be partially masked by age. The inverse relation between year of birth and Hg exposure made age an ambiguous variable. Age was a risk factor for the symptom clusters, covaried with Hg exposure, but was not a consequence of Hg exposure.
Conclusions
Since the disaster, Grassy Narrows First Nation has expressed concern about the high rate of symptoms of nervous system dysfunction in their community. This is the first study to demonstrate links between long-term and past Hg exposure and current coexisting symptoms. Given the complexity of the interrelations between the various determinants of chronic health problems in this and other First Nation communities, SEM and LMEM methods were useful to link past Hg exposure with present-day symptoms. Further studies should examine the mechanisms that underlie these manifestations.
Supplementary Information
Additional file 1: Supplementary Table 1. Characteristics of symptom clusters.
Acknowledgements
We thank all of the people of Grassy Narrows who organized and carried out the Grassy Narrows Community Health Assessment (CHA): the community advisory committee, the fieldwork coordinators and the surveyors. A special thank you to the people of Grassy Narrows who participated in the CHA and agreed to share their biomarker data with us. We salute the resilience of the Grassy Narrows community who have fought for mercury justice over the past 50 years.
Abbreviations
- Hg
mercury
- GN-CHA
Grassy Narrows Community Health Assessment
- FNRHS
First Nations Regional Health Survey
- FNIHB
First Nations and Inuit Health Branch
- SEM
Structural Equation Model
- LMEM
Longitudinal Mixed Effects Model
- CFA
Confirmatory factor analysis (CFA)
- χ2/df
Chi-square divided by the degrees of freedom
- SRMR
Standardized Root Mean Square Residual
- RMSEA
Root Mean Square Error of Approximation
- CFI
Comparative Fit Index
- TLI
Tucker Lewis Index
- AIC
Akaike’s Information Criterion
- BIC
Schwarz Bayesian Information Criterion
- DWLS
Diagonally weighted least squares
- MI
Modification Indices
- MICE
Multivariate Imputation by Chained Equations
- DAG
Directed Acyclic Graphs
- MEM
Mixed Effects Model
Authors’ contributions
AP created the database, determined the statistical approaches and performed the analyses. She co-wrote the manuscript with DM. JDS is the initiator and organizer of the GN-CHA survey. She provided input for data interpretation. MF participated in the analyses and dissemination of the GN-CHA. She participated in the writing and editing of the manuscript. TSL provided the neurological interpretation of the symptom clusters and verified the manuscript. DM was invited by Grassy Narrows First Nation as scientific advisor for the GN-CHA. She was responsible for over-seeing the fieldwork, analyzing the data and preparing the reports. She is principal investigator on the present research. All authors read and approved the final manuscript.
Funding
The GN-CHA received financial support from Health Canada and the Ontario Ministry of Health and Long-Term Care and technical support from these ministries and the Ontario Agency for Health Protection and Promotion, the Ontario Ministry of Indigenous Relations and Reconciliation and the Northwestern Health Unit of Ontario. The present study was funded by the Canadian Institutes for Health Research (#152882).
Availability of data and materials
Archived mercury biomarker data were obtained from the First Nations and Inuit Health Branch (FNIHB) of the Ministry of Indigenous Services Canada, at the request of Grassy Narrows First Nation. The datasets generated and analysed in the present study are the property of Grassy Narrows First Nation. Permission for use of the data lies with Grassy Narrows Chief and Council.
Declarations
Ethics approval and consent to participate
Ethics approval for the GN-CHA was obtained from the Manitoulin Anishinaabek Research Review Committee (MARRC), who issued an Ethics Certificate on September 1, 2016. Informed consent forms were signed by all participants. Consent included survey participation and the linking of the information from the GN-CHA to data obtained from previous surveillance programs and/or studies of Hg exposure. Ethics approval for the present study was obtained from the Université du Québec à Montréal (2016_e_1350) and Health Canada Research Ethics Board (REB 2017–0006).
Consent for publication
All participants provided consent for publication. The present study was presented to Grassy Narrows’ Chief and Council of, who approved the manuscript for publication on May 12, 2021.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rudd JWM, Turner MA, Furutani A. The English-Wabigoon River system: I. a synthesis of recent research with a view towards mercury amelioration. Can J Fish Aquat Sci. 1983;40(12):2206–2217. [Google Scholar]
- 2.Etkin D. Disaster theory: an interdisciplinary approach to concepts and causes. 1. United Kingdom: Butterworth-Heinemann, Elsevier Science and Technology; 2015. pp. 138–142. [Google Scholar]
- 3.Wheatley B, Paradis S. Exposure of Canadian Aboriginal peoples to methylmercury. Water Air Soil Pollut. 1995;80(1):3–11. [Google Scholar]
- 4.Wheatley B, Paradis S, Lassonde M, Giguere MF, Tanguay S. Exposure patterns and long term sequelae on adults and children in two Canadian indigenous communities exposed to methylmercury. Water Air Soil Pollut. 1997;97(1–2):63–73. [Google Scholar]
- 5.Neff MR, Bhavsar SP, Arhonditsis GB, Fletcher R, Jackson DA. Long-term changes in fish mercury levels in the historically impacted English-Wabigoon River system (Canada) J Environ Monit. 2012;14(9):2327–2337. doi: 10.1039/c2em30324h. [DOI] [PubMed] [Google Scholar]
- 6.Philibert A, Fillion M, Mergler D. Mercury exposure and premature mortality in the grassy narrows first nation community: a retrospective longitudinal study. Lancet Planet Health. 2020;4(4):e141–e1e8. doi: 10.1016/S2542-5196(20)30057-7. [DOI] [PubMed] [Google Scholar]
- 7.Harada M, Hanada M, Tajiri M, Inoue Y, Hotta N, Fujino T, et al. Mercury pollution in first nations groups in Ontario, Canada: 35 years of Canadian Minamata disease (English translation) J Minamata Stud. 2011;3:3–30. [Google Scholar]
- 8.Takaoka S, Fujino T, Hotta N, Ueda K, Hanada M, Tajiri M, et al. Signs and symptoms of methylmercury contamination in a First Nations community in Northwestern Ontario, Canada. Sci Total Environ. 2014;468–469:950–957. doi: 10.1016/j.scitotenv.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Eto K, Marumoto M, Takeya M. The pathology of methylmercury poisoning (Minamata disease): The 50th Anniversary of Japanese Society of Neuropathology. Neuropathology. 2010;30(5):471-9. [DOI] [PubMed]
- 10.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr. 2004;32:17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 11.Skerman HM, Yates PM, Battistutta D. Multivariate methods to identify cancer-related symptom clusters. Res Nurs Health. 2009;32(3):345-60. [DOI] [PubMed]
- 12.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manag. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Aktas A, Walsh D, Rybicki L. Symptom clusters: myth or reality? Palliat Med. 2010;24(4):373–385. doi: 10.1177/0269216310367842. [DOI] [PubMed] [Google Scholar]
- 14.Miaskowski C. Future directions in symptom cluster research. Semin Oncol Nurs. 2016;32(4):405–415. doi: 10.1016/j.soncn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, et al. Advancing Symptom Science Through Symptom Cluster Research: Expert Panel Proceedings and Recommendations. J Natl Cancer Inst. 2017;109(4):1-9. [DOI] [PMC free article] [PubMed]
- 16.Weihe P, Grandjean P, Debes F, White R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci Total Environ. 1996;186(1–2):141–148. doi: 10.1016/0048-9697(96)05094-2. [DOI] [PubMed] [Google Scholar]
- 17.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1(1):1–2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budtz-Jørgensen E, Grandjean P, Jørgensen PJ, Weihe P, Keiding N. Association between mercury concentrations in blood and hair in methylmercury-exposed subjects at different ages. Environ Res. 2004;95(3):385–393. doi: 10.1016/j.envres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez BN, Budtz-Jørgensen E, Ryan LM, Hu H. Structural Equation Models. J Am Stat Assoc. 2005;100(472):1443–1455. [Google Scholar]
- 20.Grandjean P, Budtz-Jørgensen E, Jørgensen PJ, Weihe P. Umbilical cord mercury concentration as biomarker of prenatal exposure to methylmercury. Environ Health Perspect. 2005;113(7):905–908. doi: 10.1289/ehp.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28(5):536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Steuerwald U, Debes F, Weihe P, et al. Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res. 2008;107(1):45–52. doi: 10.1016/j.envres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen TP, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117(3):367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandjean P, Weihe P, Nielsen F, Heinzow B, Debes F, Budtz-Jorgensen E. Neurobehavioral deficits at age 7 years associated with prenatal exposure to toxicants from maternal seafood diet. Neurotoxicol Teratol. 2012;34(4):466–472. doi: 10.1016/j.ntt.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex. 2016;74:358–369. doi: 10.1016/j.cortex.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FNIGC . First Nations Regional Health Survey (RHS)2008/10: National report on adults, youth and children living in First Nations communities. Ottawa: The First Nations Information Governance Centre/ Le Centre de la Gouvernance de L’information des Premères Nations; 2012. [Google Scholar]
- 28.Farant JP, Brissette D, Moncion L, Bigras L, Chartrand A. Improved cold-vapor atomic absorption technique for the microdetermination of total and inorganic mercury in biological samples. J Anal Toxicol. 1981;5(1):47–51. doi: 10.1093/jat/5.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Magos L. Selective atomic-absorption determination of inorganic mercury and methylmercury in undigested biological samples. Analyst. 1971;96(149):847–853. doi: 10.1039/an9719600847. [DOI] [PubMed] [Google Scholar]
- 30.Chavent M, Kuentz V, Liquet B, Saracco L. ClustOfVar:an R Package for the clustering of Variables. 2011. [Google Scholar]
- 31.Chavent M, Genuer R, Kuentz-Simonet V, Liquet B, Saracco J. ClustOfVar: an R package for dimension reduction via clustering of variables. Application in supervised classification and variable selection in gene expressions data. Statistical Methods for (post)-Genomics Data (SMPGD 2013); 2013-01-24; Netherlands 2013. Available from: http://www.math.u-bordeaux.fr/~mchave100p/wordpress/wp-content/uploads/2012/12/poster-SMPGD.pdf.
- 32.Kuentz-Simonet V, Lyser S, Candau J, Deuffic P. ClustOfVar-based approach for unsupervised learning: Reading of synthetic variables with sociological data. Electron J Appl Stat Anal. 2015;8:170–197. doi: 10.1285/I20705948V8N2P170. [DOI] [Google Scholar]
- 33.Kuentz-Simonet V, Labenne A, Rambonilaza T. Using ClustOfVar to construct quality of life indicators for vulnerability assessment municipality trajectories in Southwest France from 1999 to 2009. Soc Indic Res. 2017;131(3):973–997. [Google Scholar]
- 34.Xie B, Pan W, Shen X. Penalized model-based clustering with cluster-specific diagonal covariance matrices and grouped variables. Electron J Stat. 2008;2:168–212. doi: 10.1214/08-EJS194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model Multidiscip J. 1999;6(1):1–55. [Google Scholar]
- 36.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model Multidiscip J. 2001;8(3):430–457. [Google Scholar]
- 37.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res. 2006;99(6):323–338. [Google Scholar]
- 38.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods. 2008;6(1):53–60. [Google Scholar]
- 39.Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Soft. 2012;48(2):1–36. [Google Scholar]
- 40.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- 41.Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taber KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48(6):1273–1296. [Google Scholar]
- 43.Bollen KA. Latent variables in psychology and the social sciences. Annu Rev Psychol. 2002;53(1):605–634. doi: 10.1146/annurev.psych.53.100901.135239. [DOI] [PubMed] [Google Scholar]
- 44.MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annu Rev Psychol. 2000;51(1):201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- 45.Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jørgensen E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ Health. 2015;14:47. doi: 10.1186/s12940-015-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacker RE, Lomax RG. A beginner's guide to structural equation modeling: psychology press; 2004;1-510.
- 47.Shook-Sa BE, Chen DG, Zhou H. Using Structural Equation Modeling to Assess the Links between Tobacco Smoke Exposure, Volatile Organic Compounds, and Respiratory Function for Adolescents Aged 6 to 18 in the United States. Int J Environ Res Public Health. 2017;14(10):1-12. [DOI] [PMC free article] [PubMed]
- 48.Wang IE, Rhemtulla M. Power analysis for parameter estimation in Structural Equation Modeling: A discussion and tutorial. Adv Methods and Pract Psychol Sci. 2021;4(1):1-17.
- 49.Satorra A, Saris W. Power of the likelihood ratio test in covariance structure analysis. Psychometrika. 1985;50(1):83–90. [Google Scholar]
- 50.MacCallum RC, Browne MW, Cai L. Testing differences between nested covariance structure models: Power analysis and null hypotheses. Psychol Methods. 2006;11:19–35. doi: 10.1037/1082-989X.11.1.19. [DOI] [PubMed] [Google Scholar]
- 51.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1(2):130–149. [Google Scholar]
- 52.Moshagen M, Erdfelder E. A new strategy for testing structural equation models. Struct Equ Model Multidiscip J. 2016;23(1):54–60. [Google Scholar]
- 53.Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30(9):1418–1423. doi: 10.1093/ndt/gfu325. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson KD, McCann M, Katikireddi SV, Thomson H, Green MJ, Smith DJ, et al. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): a novel and systematic method for building directed acyclic graphs. Int J Epidemiol. 2019;49(1):322–329. doi: 10.1093/ije/dyz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 56.Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. 2020. [DOI] [PMC free article] [PubMed]
- 57.Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24(1):70–93. [Google Scholar]
- 59.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 60.Faul F, Erdfelder E, Buchner A, Lang A. G* Power Version 3.1. 7 [computer software] Germany: Uiversität Kiel; 2013. [Google Scholar]
- 61.Kyonka EGE. Tutorial: small-N power analysis. Perspectives on behavior science. 2018;42(1):133–152. doi: 10.1007/s40614-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Research Council Committee on the Toxicological Effects of Mercury . Toxicological effects of Methylmercury. Washington (DC): National Academies Press (US); 2000. [Google Scholar]
- 63.Wang YA, Rhemtulla M. Power analysis for parameter estimation in structural equation modeling: a discussion and tutorial. Adv Methods Pract Psychol Sci. 2021;4(1):1–17. [Google Scholar]
- 64.Chief Moon-Riley K, Copeland JL, Metz GAS, Currie CL. The biological impacts of indigenous residential school attendance on the next generation. SSM Popul Health. 2019;7:100343. doi: 10.1016/j.ssmph.2018.100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen VK. An epidemic of suspicion - Ebola and violence in the DRC. N Engl J Med. 2019;380(14):1298–1299. doi: 10.1056/NEJMp1902682. [DOI] [PubMed] [Google Scholar]
- 66.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34(5):971–980. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 67.Harada M, Hanada M, Tajiri M, Inoue Y, Hotta N, Takehiko F, et al. Mercury Poisoning in first nations groups in Ontario, Canada 35 years of Minamata disease in Canada. J Minamata Stud. 2011;3:3–30. [Google Scholar]
- 68.McAlpine D, Araki S. Minamata disease: an unusual neurological disorder caused by contaminated fish. Lancet. 1958;2(7047):629–631. doi: 10.1016/s0140-6736(58)90348-9. [DOI] [PubMed] [Google Scholar]
- 69.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 70.Ninomiya T, Ohmori H, Hashimoto K, Tsuruta K, Ekino S. Expansion of methylmercury poisoning outside of Minamata: an epidemiological study on chronic methylmercury poisoning outside of Minamata. Environ Res. 1995;70(1):47–50. doi: 10.1006/enrs.1995.1045. [DOI] [PubMed] [Google Scholar]
- 71.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 72.Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci. 2007;262(1–2):131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 73.Taber KH, Hurley RA. Mercury exposure: effects across the lifespan. J Neuropsychiatry Clin Neurosci. 2008;20(4):iv–389. doi: 10.1176/jnp.2008.20.4.iv. [DOI] [PubMed] [Google Scholar]
- 74.Yorifuji T, Tsuda T, Takao S, Harada M. Long-term exposure to methylmercury and neurologic signs in Minamata and neighboring communities. Epidemiology. 2008;19(1):3–9. doi: 10.1097/EDE.0b013e31815c09d2. [DOI] [PubMed] [Google Scholar]
- 75.Jackson AC. Chronic neurological disease due to methylmercury poisoning. Can J Neurol Sci. 2018;45(6):620–623. doi: 10.1017/cjn.2018.323. [DOI] [PubMed] [Google Scholar]
- 76.Grandjean P, Weihe P, Debes F, Choi AL, Budtz-Jørgensen E. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol Teratol. 2014;43:39–44. doi: 10.1016/j.ntt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yorifuji T, Tsuda T, Inoue S, Takao S, Harada M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environ Int. 2011;37(5):907–913. doi: 10.1016/j.envint.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120(6):799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Landrigan PJ, Kimmel CA, Correa A, Eskenazi B. Children’s health and the environment: public health issues and challenges for risk assessment. Environ Health Perspect. 2004;112(2):257–265. doi: 10.1289/ehp.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rauh VA, Horton MK, Miller RL, Whyatt RM, Perera F. Neonatology and the environment: impact of early exposure to airborne environmental toxicants on infant and child neurodevelopment. Neoreviews. 2010;11:363–369. doi: 10.1542/neo.11-7-e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heyer DB, Meredith RM. Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology. 2017;58:23–41. doi: 10.1016/j.neuro.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 82.Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996;17(3–4):583–596. [PubMed] [Google Scholar]
- 83.Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110 Suppl 5(Suppl 5):851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newland MC, Reed MN, Rasmussen E. A hypothesis about how early developmental methylmercury exposure disrupts behavior in adulthood. Behav Process. 2015;114:41–51. doi: 10.1016/j.beproc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adelson N. The embodiment of inequity: health disparities in aboriginal Canada. Can J Public Health. 2005;96 Suppl 2(Suppl 2):S45–S61. doi: 10.1007/BF03403702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ninomiya T, Imamura K, Kuwahata M, Kindaichi M, Susa M, Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicol Teratol. 2005;27(4):643–653. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Takaoka S, Fujino T, Kawakami Y, Shigeoka SI, Yorifuji T. Survey of the extent of the persisting effects of methylmercury pollution on the inhabitants around the Shiranui Sea, Japan. Toxics. 2018;6(3). [DOI] [PMC free article] [PubMed]
- 88.Yorifuji T, Takaoka S, Grandjean P. Accelerated functional losses in ageing congenital Minamata disease patients. Neurotoxicol Teratol. 2018;69:49–53. doi: 10.1016/j.ntt.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Budtz-Jørgensen E, Keiding N, Grandjean P. Effects of exposure imprecision on estimation of the benchmark dose. Risk Anal. 2004;24(6):1689–1696. doi: 10.1111/j.0272-4332.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 90.Korogi Y, Takahashi M, Okajima T, Eto K. MR findings of Minamata disease--organic mercury poisoning. J Magn Reson Imaging. 1998;8(2):308–316. doi: 10.1002/jmri.1880080210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Characteristics of symptom clusters.
Data Availability Statement
Archived mercury biomarker data were obtained from the First Nations and Inuit Health Branch (FNIHB) of the Ministry of Indigenous Services Canada, at the request of Grassy Narrows First Nation. The datasets generated and analysed in the present study are the property of Grassy Narrows First Nation. Permission for use of the data lies with Grassy Narrows Chief and Council.