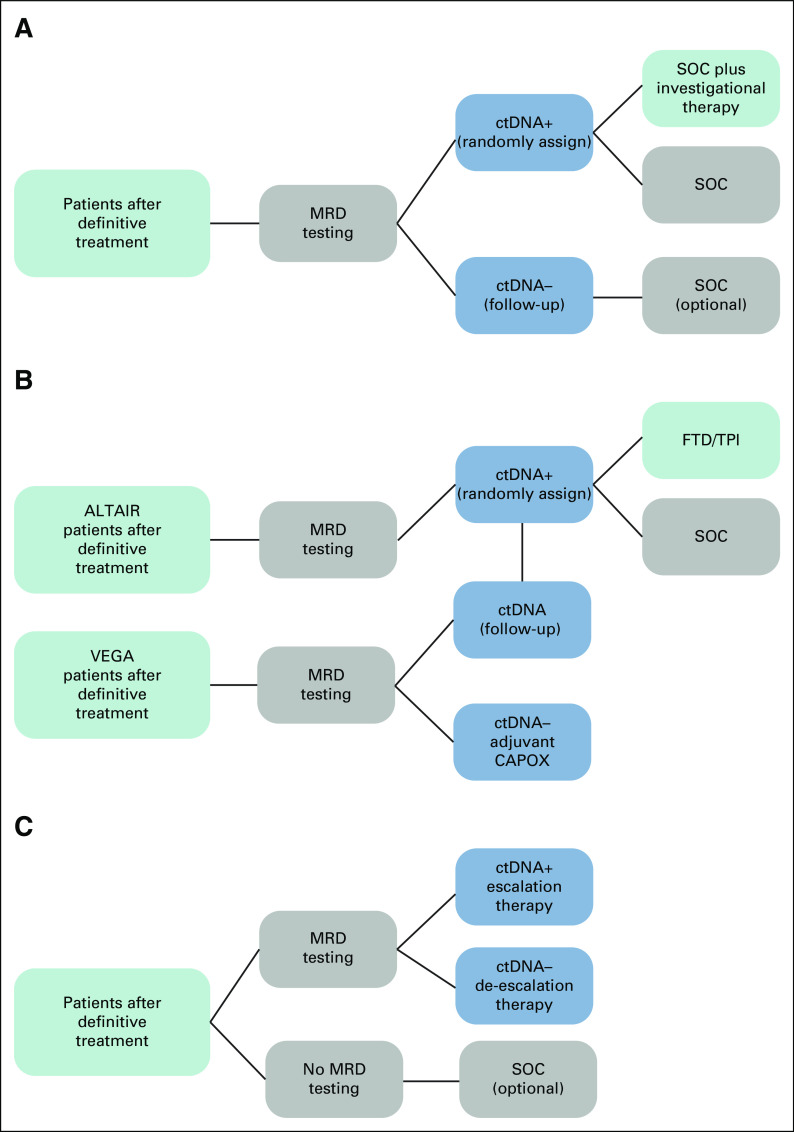
FIG 2.
(A) Marker by treatment interaction design with MRD testing after definitive treatment. ctDNA-positive patients are randomly assigned to SOC plus investigational therapy versus SOC alone. ctDNA-negative patients are assigned to the follow-up group. Noninferiority component permits comparison of ctDNA-negative patients with ctDNA-positive patients to ensure these patients have outcomes that are no worse than treatment groups. (B) Marker by treatment interaction and noninferiority designs with MRD testing after definitive treatment (GALAXY, ALTAIR, and VEGA). ctDNA-positive patients from the GALAXY study are randomly assigned in the ALTAIR study to SOC plus investigational therapy versus SOC alone. ctDNA-negative patients from GALAXY are randomly assigned to CAPOX and follow-up. Noninferiority of follow-up versus CAPOX is investigated among ctDNA-negative patients. ctDNA-negative patients from VEGA who become ctDNA-positive can crossover to ALTAIR. (C) MRD testing after definitive treatment. The results of MRD testing are used to assign ctDNA-positive patients to escalation and ctDNA-negative patients to de-escalation therapy in Arm A. Arm B has no ctDNA testing and receives SOC. ctDNA, circulating tumor DNA; FTD/TPI, trifluridine/tipiracil; MRD, molecular residual disease; SOC, standard of care.