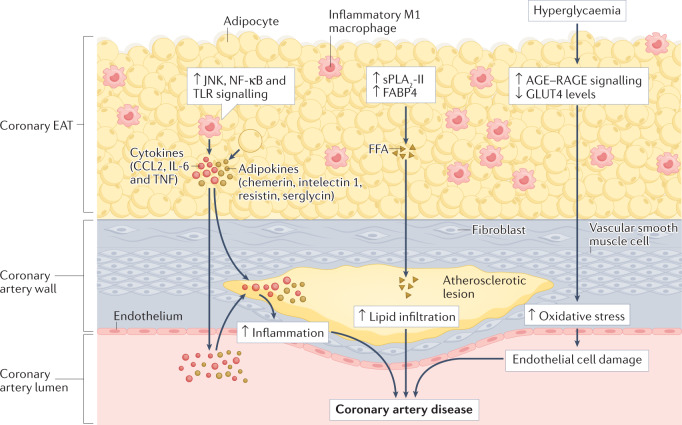
Fig. 3. Atherogenic effects of coronary EAT on the coronary artery.
In patients with coronary artery disease, coronary epicardial adipose tissue (EAT) has a dense inflammatory infiltrate with a high prevalence of pro-inflammatory M1 macrophages. Coronary EAT secretes pro-inflammatory cytokines (such as CCL2, IL-6 and tumour necrosis factor (TNF)) and adipokines (such as chemerin, intelectin 1 (also known as omentin 1), resistin and serglycin) into the coronary lumen, thereby contributing to systemic inflammation. Coronary EAT inflammation also contributes locally to coronary atherosclerotic plaque inflammation. The upregulation in the coronary EAT of innate immune response signalling, such as JUN N-terminal kinase (JNK), nuclear factor-κB (NF-κB) and Toll-like receptor (TLR) signalling, can also induce the secretion of inflammatory mediators from the coronary EAT. The excessive influx of free fatty acids (FFAs) mediated by group II secretory phospholipase A2 (sPLA2-II) and adipocyte fatty acid-binding protein (also known as FABP4) from epicardial adipocytes might infiltrate the adventitia and contribute to the lipid build-up in coronary artery atherosclerotic plaques. The co-occurrence of coronary artery disease with chronic hyperglycaemia can upregulate signalling via advanced glycation end products (AGE) binding to their receptor RAGE and reduce levels of glucose transporter type 4 (GLUT4), thereby contributing to oxidative stress and endothelial cell damage.