Fig. 3 |. A working model of the Wallerian degeneration pathway.
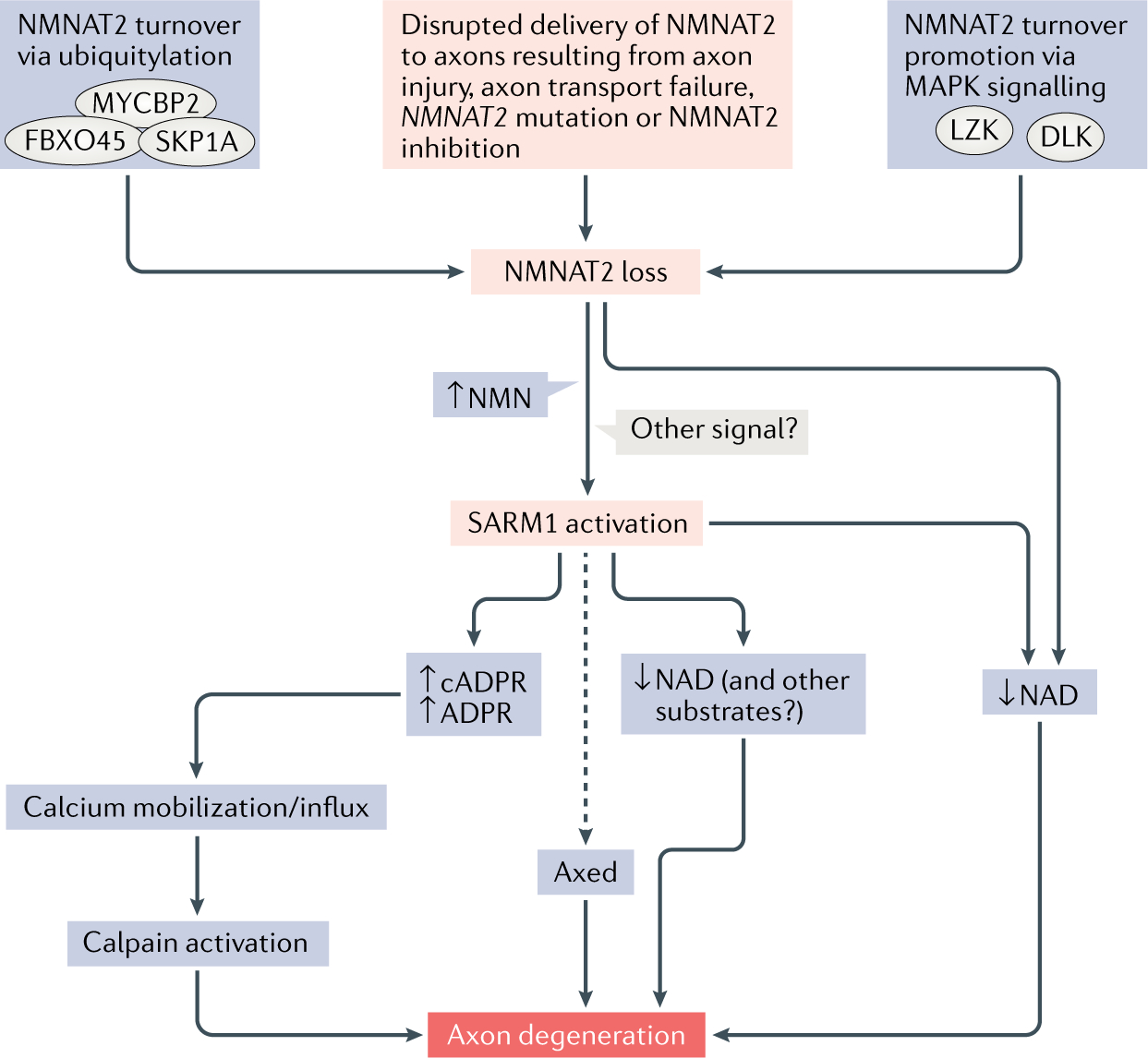
The figure shows current knowledge of the factors that determine nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) activity in axons, how NMNAT2 loss activates sterile-α and Toll/interleukin 1 receptor (TIR) motif containing protein 1 (SARM1) and events downstream of SARM1 leading to axon degeneration. Various factors disrupt the delivery of functional NMNAT2 to axons. NMNAT2 turnover is mediated by the mitogen-activated protein kinases (MAPK) dual leucine zipper kinase (DLK) and leucine zipper-bearing kinase (LZK), as well as a ubiquitin ligase complex consisting of MYC-binding protein 2 (MYCBP2), s-phase kinase-associated protein 1A (SKP1A) and F-box protein 45 (FBXO45)38. In combination, this reduces NMNAT2 levels and causes downstream activation of SARM1, probably as a result of increased levels of nicotinamide mononucleotide (NMN) but potentially also in other ways35. The combined effect of decreased NMNAT2 and increased activation of SARM1 leads to a great decrease in axonal nicotinamide adenine dinucleotide (NAD), which may itself cause axon degeneration through ATP synthesis failure25. Alternatively, other SARM1 substrates or its calcium mobilizing products could be important for the later stages of Wallerian degeneration. In Drosophila melanogaster, there is also an as-yet undefined role for Axundead (Axed) in Wallerian axon degeneration19. ADPR, ADP-ribose; cADPR, cyclic ADP-ribose.
