Abstract
Purpose:
Weight gain is common among breast cancer patients and may contribute to poorer treatment outcomes. Most programs target breast cancer survivors after the completion of therapy and focus on weight reduction. This study examined the feasibility and preliminary efficacy of an intervention designed to prevent primary weight gain among women receiving neoadjuvant chemotherapy for breast cancer.
Methods:
Thirty-eight newly diagnosed stage II or III breast cancer patients were randomized to the BALANCE intervention or usual care within three weeks of starting neoadjuvant chemotherapy. The intervention used a size acceptance-based approach, and encouraged home-based resistance and moderate-intensity aerobic exercise as well as a low energy-dense diet to prevent weight gain. Assessments were conducted at baseline, mid-chemotherapy (3 months) and post-chemotherapy (6 months). Intervention feasibility, acceptability, and preliminary effects on anthropometric, quality of life, and circulating biomarker measures were evaluated.
Results:
Intervention participant retention (100%) and in-person session attendance (80%) was high during the intervention period, although attendance dropped to 43% for telephone-delivered sessions. The majority of participants reported being satisfied with the intervention during chemotherapy (88%). Participants in the intervention group had greater reductions in waist circumference (p=.03) and greater improvements in self-reported vitality scores (p= .03) than the control group at the end of chemotherapy. Significant effects on biomarkers were not observed.
Conclusions:
A size acceptance weight management program is feasible during neoadjuvant chemotherapy among breast cancer patients, and may have beneficial effects on waist circumference and patient vitality.
Keywords: breast cancer, obesity, weight management, size acceptance, survivorship
BACKGROUND
Weight gain is a common problem among women diagnosed with breast cancer [1], particularly for women who are normal weight or overweight at diagnosis [2,3], younger women [4–6], and those receiving chemotherapy [7,4]. Most weight gain is observed in the first year after diagnosis [8]. Weight gain takes place throughout the course of chemotherapy [3,2,9–16], continues after treatment ends [9,10,17,18,16,19,4], and is greater than that observed in healthy women as they age [7,20].
Weight gain has a negative effect on the well-being of breast cancer patients and may influence cancer-specific and non-cancer-related health outcomes. Weight gain is distressing and can result in negative body image [21–23]. Breast cancer survivors who gain weight report poorer physical functioning, lower vitality, and more role limitations due to physical symptoms [24]. Several studies indicate that weight gain after diagnosis increases risk of all-cause mortality [25]. In a large prospective US cohort, the Nurses’ Health Study, weight gain after diagnosis was associated with an increased risk of recurrence, breast cancer death, and total mortality among never smokers, those with BMI <25 at baseline, women with tumors ≤ 2 cm, and those with no nodal involvement [26].
Most weight management trials among breast cancer populations focus on weight loss after it is gained [27], as opposed to targeting weight gain prevention. Weight loss interventions have been shown to be safe and feasible for breast cancer survivors recruited after the completion of treatment [27]. However, less is known about the feasibility of weight gain prevention interventions during chemotherapy, a time when patients are likely to initiate weight gain. A recent review of weight gain prevention interventions during active breast cancer treatment noted major limitations in the current research including small numbers of studies, and infrequent use of blood biomarkers in analysis [28].
The current pilot study assessed the feasibility and acceptability of conducting a randomized behavioral weight gain prevention intervention during neoadjuvant chemotherapy, and evaluated its effects on weight, waist circumference, and quality of life. Additionally, blood biomarkers related to adiposity and known to influence breast cancer prognosis, including sex hormone-binding globulin (SHBG), fasting insulin and glucose, IGF-I, and IGFBP-3, adiponectin, and leptin were assessed. A-priori feasibility and acceptability targets were not set for this study, but rather were examined broadly in order to identify potential for a larger trial. We hypothesized that the intervention would be feasible and acceptable to patients, and would positively affect body weight, waist circumference, quality of life, and biological markers.
METHODS
This study was a randomized pilot test of the Project BALANCE (Balancing Activity, Lifestyle And Nutrition throughout the Cancer Experience) weight gain prevention intervention (www.clinicaltrials.gov, NCT00533338). The University of Texas MD Anderson Cancer Center (UTMDACC) institutional review board approved this protocol (2005–0888). Written informed consent was collected from all participants. CONSORT guidelines were followed in the reporting of this trial.
Participants
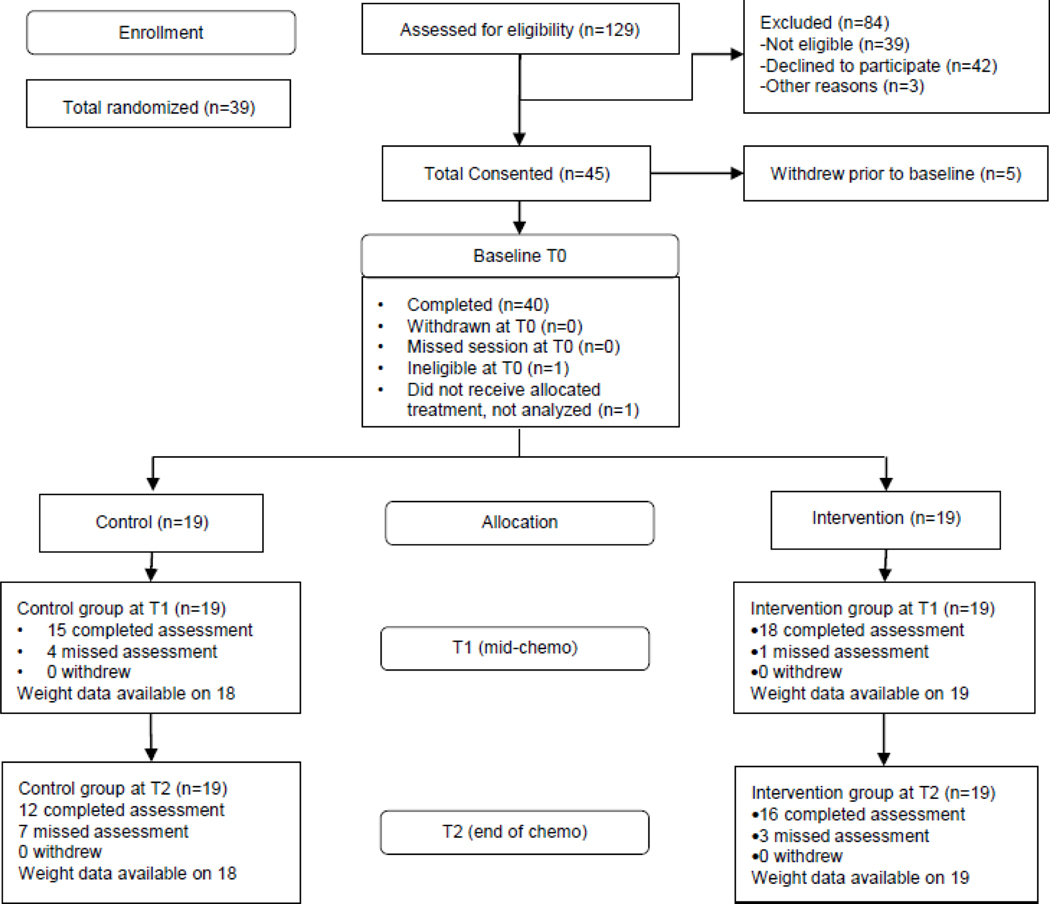
Participants were recruited at UTMDACC. Eligibility criteria included 1) newly diagnosed stage II or III breast cancer patients within 3 weeks of starting neoadjuvant chemotherapy, 2) ability to speak and read English, 3) cognitive ability to give informed consent, 4) willingness to come to UTMDACC once a week, 5) reside in Harris county or a contiguous county, and 6) have physician clearance to engage in unsupervised physical activity. Patients with absolute or relative contraindications for exercise testing, as defined by the American College of Sport Medicine [29], were excluded as were patients with uncontrolled asthma or other medical contraindications as defined by the patient’s physician. Patients with inflammatory breast cancer were also excluded. A total of 129 patients were assessed for eligibility (Figure 1). Thirty-nine patients (30%) were ineligible based on the criteria above, and 42 patients (33%) declined to participate. Thirty-eight participants were randomized and 37 completed the study.
Fig 1:
CONSORT diagram for Project BALANCE
Procedures
Eligible patients were identified by providers or study staff using UTMDACC’s electronic medical record. After identification, a research coordinator met with potential participants during previously scheduled appointments or by phone to describe the study and assess interest. Physicians were required to sign a release form to indicate the participant was medically eligible. Enrolled participants provided written informed consent and completed baseline assessments including anthropometrics, serum collection, and questionnaires related to demographics, body image (Body Image Scale) and quality of life (Medical Outcomes Study (SF-36)).
Following baseline, participants were randomized to Project BALANCE or the usual care control group. A form of adaptive randomization called minimization, which is similar to stratification in that participant characteristics are used to assign them to the treatment conditions [30], was used to ensure group balance with respect to participant characteristics. Covariates considered in the randomization of participants to study condition included baseline BMI (median), age (median), disease stage (I / II), menopausal status (yes / no), treatment regimen (short interval/long interval), physical activity (meets standard guidelines / does not meet standard guidelines), and vegetable consumption (high / low as per questionnaire).
All participants completed questionnaires and anthropometric measurements at baseline (T0), mid-chemotherapy three months later (T1) and post-chemotherapy six months after baseline (T2). Serum was collected at T0 and T2 only.
Intervention conditions
Project BALANCE
Recognizing that women undergoing breast cancer treatment are at particularly heightened risk of having body image concerns [31–33], the intervention used a size acceptance-based approach. The size acceptance approach to weight management has shown effectiveness at maintaining weight and improving cardiovascular risk factors [34,35] and resonates well with middle-aged women [34]. It focuses on improving health through lifestyle change, and de-emphasizes weight loss. The goal of Project BALANCE was to provide information, increase behavioral capability, and enhance self-efficacy in four areas stressed by the size acceptance approach: body acceptance, eating behavior, nutrition, and activity. Project BALANCE materials focus on teaching women to disentangle feelings of self-worth from weight and to develop realistic expectations about body size and shape. Intervention materials include modeling of body acceptance through stories of women with breast cancer coping with the physical changes that result from treatment.
Participants received the intervention via 20 individual counseling sessions over approximately 6 months during chemotherapy (14 in-person and 6 by telephone). Participants attended one 60-minute session per week for 13 weeks, where they performed the resistance and flexibility exercises and received corrective feedback; reviewed the previous week’s food records; discussed progress and barriers related to exercise and diet goals; and set goals for next week. In-person appointments were typically scheduled on the same day participants received chemotherapy. Following the in-person sessions, participants received one weekly telephone session for the following 6 weeks of chemotherapy. Phone calls were scheduled with research staff at the participant’s convenience. A final in-person session was conducted at the end of chemotherapy. All sessions were conducted with masters-level health educators.
The dietary aspect of Project BALANCE focused on promoting foods with low energy density, which have been shown to increase satiety more than high energy density foods with the same caloric value [36–38]. This approach was selected as low energy dense foods are low in fat and high in water and fiber, thus emphasizing a diet consistent with published recommendations for survivors [39]. The intervention counseling sessions focused on developing skills for internal regulation and mindful eating. Study participants were asked to keep diet records on at least one weekday and one weekend day to keep track of food eaten, time eating, and mood while eating during the entire intervention.
The exercise aspect of the intervention focused on resistance and flexibility training to maintain or increase lean body mass. Each participant was prescribed tailored resistance and flexibility exercises for the major muscle groups based on a baseline strength assessment. Lighter weight and more repetition rather than heavier weight and less repetition was emphasized during exercise sessions. Participants were asked to perform the exercises 3 times per week including the weekly supervised session. Exercise sessions targeted major muscle groups and generally proceeded from core muscle groups (torso), to large muscle groups (quadriceps, semitendinosis, biceps femoris, gastrocnemius, soleus, biceps, triceps) and then with smaller muscle groups (brachoradialis, deltoids, shoulder girdle muscle, etc). Flexibility exercises included all joint areas but focused mainly on areas being resistance trained and areas with less range of motion. Specific flexibility exercises were prescribed based on assessment results, resistance training progress, and individual needs.
Participants were asked to maintain levels of aerobic activity. Baseline levels of activity were established with a pedometer during a one-week baseline period. Participants were then asked to continue wearing the pedometer and recording steps, with the goal of maintaining the baseline number of steps per week throughout the six months of chemotherapy. Because fatigue or other treatment-related symptoms may cause patients to decrease usual occupational and household activities, participants were encouraged to take brief moderate intensity walks to maintain baseline activity levels and avoid transitioning into sedentary activity patterns.
Project BALANCE also included written materials (including newsletters and instructions developed specifically for this study plus the Volumetrics cookbook [40]) and exercise equipment (hand-held weights, steps, stability balls and pedometers). The written materials incorporated Social Cognitive Theory constructs to provide information and skills necessary to support behavior change. For example, the materials included photos illustrating how to properly perform exercises, charts for monitoring progress, low-energy-density recipes, recommended shopping lists, and tips on choosing foods when eating out. In addition, they included stories about other cancer survivors who had made positive diet and activity changes in their lives, to increase self-efficacy through modeling. To encourage size acceptance, the materials included illustrations and photographs of people with a range of body sizes, and stories on developing a healthy body image and coping with appearance changes after breast cancer diagnosis. The materials also provided information on behavioral skills such as overcoming barriers, rewarding yourself, and time management.
Usual care
Participants received publically available written materials on cancer survivorship only. These materials address concerns of breast cancer survivors and provide basic information about appropriate diet and exercise during chemotherapy, but do not provide individually tailored recommendations or behavior change skills.
Measures
Feasibility and Acceptability were assessed by attrition (remaining in the study), adherence to intervention (completing study sessions), and satisfaction (based on self-reported satisfaction at T2 and likelihood of recommending the intervention to a friend).
Questionnaires included a demographic questionnaire covering questions on race, medical history, age, education, employment, marital status, and household. Body Image was assessed using the self-report Body Image Scale (BIS). The BIS is a 10-item questionnaire measuring body image concerns resulting from cancer and cancer treatment, with higher scores indicating more body image distress; it has demonstrated validity and reliability among cancer patients [41]. Health-related quality of life was assessed using The Medical Outcomes Study (SF-36) US Standard Version 1.0. Internal consistency reliability is high for the SF-36’s 8 scales (physical functioning, role functioning, bodily pain, general health, vitality, social functioning, role emotional and mental health) that range from 0 (lowest level of functioning) to 100 (highest level of functioning) [42]. Questionnaires were administered to participants during in-person assessments. If participants needed additional time to complete the questionnaires they were allowed to take them home and return by mail or at a subsequent appointment.
Anthropometrics included height and weight measured in light clothing and without shoes at in-person visits using an electronic scale and stadiometer. If a participant missed an assessment, their weight was obtained from the medical record as measured at the clinic visit closest to the scheduled assessment. Waist circumference was measured at the narrowest part of the torso using a Gulick flexible tape.
Biomarkers were assessed from serum samples collected after an overnight fast and stored in a −80-degree freezer until analyzed. Quantitative enzyme-linked immunosorbent assay (ELISA) was conducted using various kits, which included Estradiol Ultrasensitive (20-ESTHUU-E01; Alpco Diagnostics, Salem NH), Sex Hormone Binding Globulin (SHBG; 11-SHBHU-E01; Alpco Diagnostics, Salem NH), IGF-1 (11-SHBHU-E01; Alpco Diagnostics, Salem NH), IGFBP-3 (DGB300; R&D Systems, Inc, Minneapolis, MN), Leptin (EZHL-80SK; EMD Millipore, St. Charles, Missouri), and Adiponectin (EZHADP-61K; EMD Millipore, St. Charles, Missouri). Results were quantified according to manufacturer’s guidelines using a Wallac VICTOR3™ Multilabel Plate Reader (PerkinElmer, Shelton, CT).
Statistical analysis
Because this was primarily a descriptive and exploratory feasibility study, a power analysis was not conducted. Given the developmental nature of the study as well as the small sample size, we did not expect to have sufficient power to detect a small or moderate effect for anthropometric or biological outcomes. Instead, findings from this study will be used for justification of further investigation on the weight gain prevention program; and the estimation of effect sizes may be helpful for planning future larger studies. Baseline characteristics of the sample were compared between participants in the intervention and usual care groups using Fisher’s exact and t-tests. Participant attrition was calculated as percentage of total randomized participants that remained in study (did not withdraw). Adherence to intervention was determined by examining the percentage of total participants that completed study sessions.
Secondary study aims were explored primarily using intent-to-treat analysis with worst observation carried forward (WOCF) imputation for all participants. As such, missing values for waist circumference or weight were substituted with a highest measurement across the time points. A sensitivity analysis using observed data only (no imputation) was also conducted. Differences between participants in the intervention and usual care groups for weight, waist circumference, quality of life, body image, and levels of biomarkers were assessed using linear mixed models. Linear mixed models with random intercepts were used, which allowed for subject-specific response trajectories and accounted for the correlations among observations arising from the same individuals over time.
Biomarker data were examined for all participants with WOCF imputation. Changes in biomarkers from baseline (T0) to end of chemotherapy (T2) were assessed using paired t-tests in each group. All tests were conducted at a two-sided significance level of .05. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Participants and group comparability
Table 1 provides the characteristics of the randomized sample. The sample was predominantly white or African-American, with high levels of education. The majority of participants were overweight or obese, and just over half were post-menopausal at diagnosis. The most common treatment regime included 12 weekly cycles of paclitaxel followed by 4 cycles (every 21 days) of 5-fluorouracil, Adriamycin/epirubicin, and cyclophosphamide (FAC or FEC) with or without trastuzumab. There were no significant group differences with regard to demographic or clinical characteristics, body composition, patient-reported outcomes, or biomarkers at baseline. Figure 1 depicts the study CONSORT diagram highlighting the percentage of eligible patients who agreed to participate (42%) and the percent completing each assessment.
Table 1:
Characteristics of the sample
| Characteristic | Intervention (n=19) % (N) | Control (n=18) % (N) | aP-value |
|---|---|---|---|
| Race/ethnicity | |||
| African-American | 26.3% (5) | 16.7% (3) | 0.863 |
| Hispanic, white | 0% (0) | 0% (0) | |
| White, non-Hispanic | 52.6% (10) | 66.7% (12) | |
| Asian | 10.5% (2) | 5.6% (1) | |
| Other | 10.5% (2) | 11.1% (2) | |
| Education | |||
| Less than high school | 5.3% (1) | 5.6% (1) | 0.903 |
| High school degree | 5.3% (1) | 5.6% (1) | |
| Some post-high school | 42.1% (8) | 38.9% (7) | |
| Bachelor’s degree | 31.6% (6) | 44.4% (8) | |
| Advanced degree | 15.8% (3) | 5.6% (1) | |
| Marital status | |||
| Single, never married | 26.3% (5) | 5.6% (1) | 0.154 |
| Married/in committed relationship | 47.4% (9) | 72.2% (13) | |
| Divorced / Widowed | 26.3% (5) | 22.3% (4) | |
| Baseline BMI | |||
| <25 | 21.1% (4) | 27.8% (5) | 0.913 |
| 25–29.9 | 26.3% (5) | 27.8% (5) | |
| ≥30 | 52.6% (10) | 44.4% (8) | |
| Menopausal status | |||
| Premenopausal | 42.1% (8) | 44.4% (8) | >.999 |
| Postmenopausal | 57.9% (11) | 55.6% (10) | |
| Estrogen Receptor Positive | 57.9% (11) | 58.8% (10) | .995 |
| Progesterone Receptor Positive | 52.6% (10) | 35.3% (6) | .335 |
| HER2 Neu Positive | 52.6% (10) | 35.3% (6) | .219 |
| Triple Negative (Yes) | 10.5% (2) | 11.1% (2) | .481 |
| Disease Stage | |||
| II | 73.7% (14) | 72.2% (13) | |
| III | 26.3% (5) | 27.8% (5) | >.999 |
| Chemotherapy Protocol | |||
| Paclitaxel + FAC/FEC | 79.0% (15) | 66.7% (12) | .468 |
| Paclitaxel + FAC/FEC + trastuzumab | 10.5% (2) | 27.8% (5) | |
| Other | 10.5% (2) | 5.6% (1) | |
| Radiation | |||
| No | 15.8% (3) | 33.3% (6) | .269 |
| Yes | 84.2% (16) | 66.7% (12) | |
| Additional Cancer Dx (other than breast) | |||
| No | 89.5% (17) | 88.9% (16) | >.999 |
| Yes | 10.5% (2) | 11.1% (2) | |
| Mean (SD) | Mean (SD) | ||
| Age | 49.6 (13.3) | 49.2 (9.2) | .925 |
Fisher’s exact tests (two-sided) were used to examine associations between intervention groups and population characteristics for categorical variables. For continuous variables, t-tests were used to test for differences between the intervention groups.
Feasibility and Acceptability
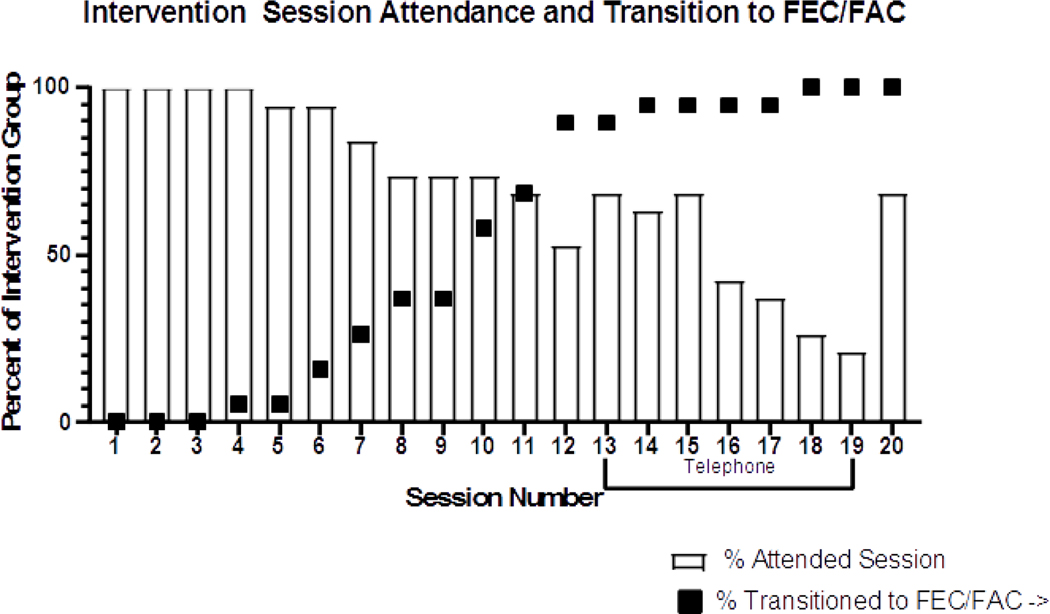
Patient retention in the intervention group was 100%, with no participants withdrawing from study for any reason. However, some participants failed to complete all study measures, with 5% (n=1) and 16% (n=3) missing assessment sessions at mid-chemotherapy and post-chemotherapy time points, respectively. Intervention participants attended an average of 80% of the in-person sessions and 43% of the telephone sessions during chemotherapy (Figure 2). In the control group, 5% (n=1) withdrew from the study; 19% (n=4) and 38% (n=7) missed the mid-chemotherapy and post-chemotherapy assessments, respectively. The intervention group reported high levels of satisfaction; 88% (n=8) of participants reported at T2 that they were satisfied with Project BALANCE, and 88% (n=8) said they would be likely to recommend it to a friend. Two optional open-ended questions were included on final surveys. Responses to the question “What did you like about the study” included the study materials and information (n= 3) and the study staff (n=1). In response to the question “What changes would you recommend?” participants noted Spanish materials (n=1), improved questionnaires (n=1) and a fully equipped and staffed gym (n=1).
Fig 2:
Scheme depicting intervention session attendance and transition from weekly treatment (12 cycles paclitaxel) to treatment every 3 weeks (4 cycles 5-fluorouracil, Adriamycin/epirubicin, and cyclophosphamide (FAC or FEC) with or without trastuzumab) among Project BALANCE participants.
Preliminary Effect on Anthropometrics
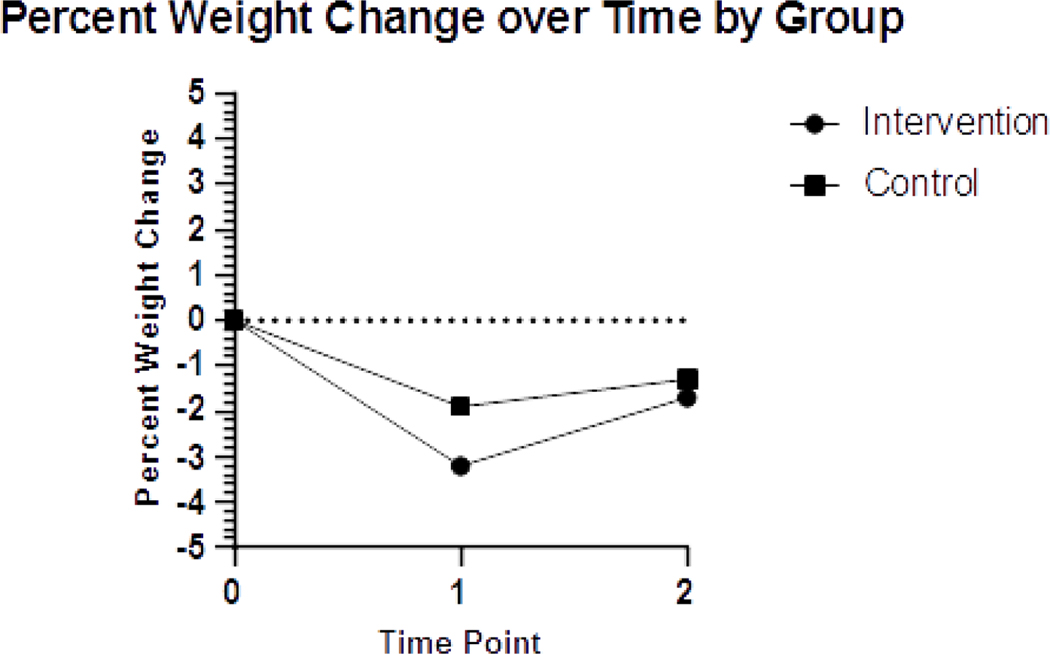
Table 2 shows estimated intervention effects on anthropometric and quality of life variables. Using intent-to-treat analysis, the intervention group weighed less across T1 and T2 time points compared to control participants but between group changes due to the intervention were not significant (p = .083). Intervention participant weight at baseline (mean = 76.0kg +/− 15.5) was reduced by mid-chemotherapy (73.5kg +/− 15.4) with some weight regain seen at the end of chemotherapy (74.8kg +/− 16.9). This trend also held true for the control group with weight fluctuating slightly from baseline (76.9kg +/− 15.5) to mid-chemotherapy (75.4kg +/− 15.0) to end of chemotherapy (75.8kg +/− 15). This finding is shown in terms of percent body weight lost in Figure 3. Waist circumference was significantly lower across time points T1-T2 in the intervention group compared to control (p= .019). Estimated intervention effects were similar using observed measures only (no imputation) versus the WOCF approach for weight (−2.66 (SE = 1.26) vs −2.01 (SE = 1.15)) and waist circumference (−3.03 (SE = 1.52) vs −3.27 (SE = 1.37)).
Table 2:
Intervention effects on weight, waist circumference, and quality of life and body image
| Baseline (T0) Mean(SD) | Change T1-T0 Mean(SD) | Change T2-T1 Mean(SD) | Adjusted Mean (SE)b | Estimated Intervention Effect (SE)a | P-value | |
|---|---|---|---|---|---|---|
| Weight (kg) | −2.01 (1.15) | 0.083 | ||||
| Intervention | 74.23 (15.54) | −0.5 (3.65) | −2.59 (2.88) | 73.53 (0.83) | ||
| Usual Care | 75.61 (15.26) | 1.32 (1.8) | −1.54 (2.32) | 75.54 (0.78) | ||
| Waist Circumference (cm) | −3.27 (1.37) | 0.019* | ||||
| Intervention | 89.27 (13.59) | −1.4 (5.08) | −0.51 (4.64) | 86.66 (1.00) | ||
| Usual Care | 87.13 (12.15) | 2.21 (4.88) | −0.41 (2.39) | 89.94 (0.94) | ||
| Quality of Life (SF-36) c | ||||||
| Physical Functioning | 4.46 (7.50) | 0.554 | ||||
| Intervention | 66.33 (33.51) | −5.77 (28.78) | 10 (17.16) | 65.63 (5.08) | ||
| Usual Care | 80.76 (19.69) | −21.07 (25.73) | 5.2 (22.61) | 69.13 (5.35) | ||
| Mental Health | −5.32 (3.77) | 0.163 | ||||
| Intervention | 67 (18.45) | .29 (17.64) | −2 (14.39) | 73.66 (2.66) | ||
| Usual Care | 75.11 (17.76) | 4.57 (10.74) | 3.64 (6.31) | 78.98 (2.56) | ||
| Vitality | 14.14 (4.84) | 0.005** | ||||
| Intervention | 41.56 (17.96) | .48 (16.74) | 7.83 (19.55) | 53.66 (3.24) | ||
| Usual Care | 57.5 (19.72) | −22.14 (17.94) | 10.91 (18.82) | 39.53 (3.19) | ||
| Role Physical | 9.38 (9.38) | 0.321 | ||||
| Intervention | 40 (40.97) | 9.62 (42.74) | 0.83 (46.22) | 46.28 (6.85) | ||
| Usual Care | 45.83 (46.38) | −16.07 (73.78) | 4.55 (47.19) | 36.91 (6.45) | ||
| Bodily Pain | 5.42 (5.60) | 0.337 | ||||
| Intervention | 63.93 (34.36) | .08 (28.41) | 2.5 (35.92) | 64.42 (4.11) | ||
| Usual Care | 63 (22.9) | −7.57 (21.87) | 1.18 (19.18) | 59.00 (3.83) | ||
| General Health | 4.93 (5.09) | 0.337 | ||||
| Intervention | 61.83 (18.15) | −.59 (15.76) | −0.2 (17.4) | 68.63 (3.56) | ||
| Usual Care | 69.61 (16.39) | −11.3 (25.16) | 6.93 (24.65) | 63.70 (3.48) | ||
| Social Functioning | 2.66 (5.51) | 0.631 | ||||
| Intervention | 55 (27.87) | 4.81 (18.07) | 12.5 (31.73) | 70.81 (3.93) | ||
| Usual Care | 70.14 (28.81) | −10.71 (21.85) | 13.64 (19.73) | 68.15 (3.70) | ||
| Role Emotional | 0.27 (11.92) | 0.598 | ||||
| Intervention | 52.22 (39.27) | 19.23 (47.06) | 10 (35.31) | 69.54 (8.65) | ||
| Usual Care | 66.67 (41.22) | 2.38 (56.18) | 0 (42.16) | 69.28 (8.03) | ||
| Body Image Scale (BIS) d | −3.00 (2.23) | 0.184 | ||||
| Intervention | 29.63 (6.83) | −.21 (1.47) | −1.03 (1.1) | 8.34 (1.60) | ||
| Usual Care | 28.62 (5.62) | .5 (.71) | −.6 (.91) | (1.56) |
Effect of intervention across the study period were analyzed using linear mixed models with random intercepts. The estimated effect represents average change in the outcome variable due to intervention across the study period, adjusting for the baseline level. Standard errors (SE) of the estimates are provided in parentheses.
Represents the expected value of the outcome across the study period estimated by the linear mixed model, for each intervention groups adjusting for the baseline level. Standard errors (SE) of the estimates are provided in parentheses.
Higher score on the SF-36 sub-scales indicates less disability.
Higher score on the BIS indicates more unfavorable body attitude
p < .05
p <.01
Fig 3:
Graph depicting percent weight loss over the study period by group
Changes in Quality of Life and Body Image
Exploratory effects of the intervention on quality of life and body image are shown in Table 2. The intervention group reported significantly greater improvements on the SF-36 vitality subscale than control participants at the end of chemotherapy (M=53.66, SE=3.24 versus M=39.53, SE=3.19, p=.005). Differences in other SF-36 scores and the BIS were not significant.
Changes in Biomarkers
There were no significant differences in changes in biomarkers between the intervention and control group (Table 3). The intervention group had significant increases in SHBG (p<.001) from baseline to T2. The control participants had significant increases in insulin (p=.004) from baseline to T2. Only a small number of participants had estradiol levels that were detectable using the ELISA. Therefore, estradiol differences between groups were not explored.
Table 3.
Change in biomarkers from baseline to end of chemotherapy N=37
| Within group change | Between group change | ||||
|---|---|---|---|---|---|
| Biomarkera | Mean (SD) | T | P-value | T | P-value |
| SHBG | |||||
| Intervention | 0.29(0.18) | 6.02 | <.001** | 1.78 | 0.087 |
| Usual care | 0.1(0.39) | 1.11 | 0.281 | ||
| Estradiol b | |||||
| Intervention | −0.59(1.6) | −1.23 | 0.248 | — | — |
| Usual care | — | — | — | — | — |
| IGF-I | |||||
| Intervention | 0.1(0.41) | 0.93 | 0.367 | −0.24 | 0.810 |
| Usual care | 0.14(0.35) | 1.68 | 0.111 | ||
| IGFBP-3 | |||||
| Intervention | 0.08(0.33) | 0.88 | 0.393 | 1.04 | 0.306 |
| Usual care | −0.05(0.34) | −0.58 | 0.571 | ||
| Insulin c | |||||
| Intervention | 0.14(1.05) | 0.50 | 0.623 | −1.23 | 0.233 |
| Usual care | 0.54(0.69) | 3.32 | 0.004** | ||
| Glucose c | |||||
| Intervention | 0.07(0.24) | 1.08 | 0.299 | 0.59 | 0.557 |
| Usual care | 0(0.37) | 0.05 | 0.961 | ||
| Adiponectin | |||||
| Intervention | −0.17(0.31) | −1.76 | 0.109 | 0.73 | 0.496 |
| Usual care | −0.33(0.46) | −1.6 | 0.186 | ||
| Leptin | |||||
| Intervention | 0(0.18) | 0.05 | 0.961 | −1.18 | 0.266 |
| Usual care | 0.1(0.15) | 1.59 | 0.187 | ||
all biomarkers studied were natural log transformed
Estradiol values were all zero for the usual care group for all time points. Only one participant in the usual care group reported still having menstrual cycles.
Glucose and Insulin were measured in mmol/L and pmol/L
significant at p< .05
significant at p< .01
DISCUSSION
This randomized pilot study indicates that a size acceptance-based weight management program delivered during neoadjuvant chemotherapy merits further investigation in a larger, fully-powered trial. Preliminary analysis shows the intervention is acceptable to participants, can prevent weight gain and even promote modest weight loss, and has a positive effect on patient vitality and waist circumference among breast cancer patients. This pilot study adds to the small but growing literature indicating weight control interventions are feasible and beneficial for breast cancer patients during treatment [28].
Project BALANCE participants had high attendance at in-person sessions during chemotherapy, but lower adherence to telephone sessions, potentially due to their treatment schedule. The majority of the patients were receiving 12 weekly cycles of paclitaxel followed by 4 cycles (every 21 days) of FAC or FEC. Most of the in-person sessions were conducted when participants were coming into the hospital weekly, with Project BALANCE appointments typically scheduled on the same day they received chemotherapy. Once the patients transitioned to FEC/FAC, they came to the hospital less frequently and also reported more side effects, potentially making adherence more difficult (Figure 2). A review of weight gain prevention studies among breast cancer patients noted successful interventions were more likely to use frequent physical contact with participants [28]. Because attendance was greater for in-person counseling sessions delivered at the time of chemotherapy appointments, future iterations of Project BALANCE should consider concentrating intervention sessions during the in-clinic phase of treatment to optimize adherence. Although adherence to telephone sessions was lower than in-person sessions, more digitized intervention delivery methods, such as text messaging, mobile apps, and social media groups, may support participant engagement during the transition to home-based care [43].
Although not statistically significant, intervention participants weighed less than control group participants at the end of chemotherapy and lost a larger percentage of body weight over the intervention period. Beyond weight, our study demonstrated a significant effect on waist circumference. An elevated waist circumference, which may indicate greater visceral adiposity, is associated with more adverse health outcomes, such as cardiovascular disease, diabetes, and colorectal and post-menopausal breast cancer [44–46], and central adiposity has been shown to be related to poorer overall and disease-specific survival in breast cancer survivors [47].
These anthropometric results are comparable to other post-treatment weight loss interventions for breast cancer survivors. One systematic review [27] identified 10 randomized trials and four single-arm trials of weight loss interventions for breast cancer survivors. Thirteen out of 14 trials showed statistically significant weight loss, with losses ranging from 1.1 kg to 11.6 kg. Another smaller review of weight gain prevention interventions during active breast cancer therapy, noted similar results to the current study. In that review, five completed interventions and five ongoing trials were included. Of the seven intervention arms examined, two achieved weight losses, and two reported successful weight maintenance [28]. The authors concluded weight gain prevention interventions during chemotherapy were feasible and generally effective at preventing weight gain among breast cancer patients. Given the difficulty most people have with maintaining weight loss over time [48], prevention of primary weight gain may be a more effective strategy for this population [28].
The size-acceptance approach was a unique aspect of this study. A recent systematic review examined the impact of ‘health, not weight loss, focused’ weight loss interventions compared to conventional weight loss programs on cardiovascular disease risk factors and body weight among overweight and obese adults. The meta-analysis of eight studies found that although mean weight loss was greater in the conventional weight loss groups at the end of treatment, those in the ‘health, not weight loss, focused’ group had more favorable improvements in weight after a longer follow up period (53 to 104 weeks) [35]. Future iterations of Project BALANCE should consider longer follow-up periods in order to better understand the maintenance of effects.
Project BALANCE had a positive impact on fatigue, as indicated by the vitality subscale of the SF-36, a validated measure of cancer-related fatigue [49]. This finding is in line with previous studies. Reeves et al demonstrated a significant improvement in fatigue after participation in a telephone-based diet and exercise study among women that recently completed breast cancer therapy [50]. Fatigue is a highly prevalent side effect of chemotherapy that can significantly impair quality of life and functioning, and has been associated with higher BMI among breast cancer survivors [51–53]. The integration of other measures of fatigue and related conditions, such as sleep quality, into a future randomized trial may help further elucidate the impact of weight loss interventions on this important side effect of cancer therapy.
This study has several strengths including the use of a body size- acceptance model and integration of multiple outcome measures of adiposity, biological markers and quality of life. Project BALANCE focused on healthy behavior change and emphasizing positive body image instead of highlighting weight loss. It is worth noting that the intervention appeared to have a small positive impact on body image, and this outcome warrants further exploration in future, fully powered trials.
This study had several limitations. The small sample was overall highly educated, and therefore may not be representative of the general breast cancer population. Additional research in a community hospital serving a more diverse population may be warranted. This study was not sufficiently powered to determine effects on biological, anthropometric or self-reported participant outcomes, although many areas for potential exploration in future studies were identified. Waist circumference may be subject to measurement error. These limitations are consistent with the extant literature on weight gain prevention among breast cancer patients on active therapy, which is generally limited to pilot studies with small sample sizes and little post-intervention follow-up.[28] Fully powered, long term trials are needed to test the preliminary findings of this emerging area of research.
CONCLUSIONS
Our findings indicate that implementing a weight gain prevention intervention among breast cancer patients during treatment is feasible and that using a size acceptance-based approach was well-received. Preliminary efficacy findings further suggest positive effects on anthropometric and quality of life outcomes that could prevent or limit future weight gain and help patients manage treatment-related symptoms. Future research is warranted to test similar interventions, particularly those that leverage the frequency of physical patient interactions occurring during treatment, to address weight control in this population.
Acknowledgements:
Work described was supported by the Lance Armstrong Foundation, the PROSPR Shared Resource (P30 CA016672), and the Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment and the Cancer Prevention Research Training Program.
Footnotes
Compliance with Ethical Standards:
This study was registered as a clinical trial at (www.clinicaltrials.gov, NCT00533338).
All procedures performed in studies involving human participants were in accordance with the ethical standards of The University of Texas MD Anderson Cancer Center (UTMDACC) institutional review board (2005–0888) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was collected from all individual participants included in the study.
Conflicts of interest: No conflicts of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Berg M, Winkels R, Kruif JTC, Laarhoven H, Visser M, Vries J, Vries Y, Kampman E (2017) Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC cancer 17 (1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen MJ, Shapiro A, Swenson KK (2011) Changes in weight and body composition in women receiving chemotherapy for breast cancer. Clin Breast Cancer 11 (1):52–60. doi: 10.3816/CBC.2011.n.009 [DOI] [PubMed] [Google Scholar]
- 3.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, Weltzien E (2008) Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19 (10):1319–1328. doi: 10.1007/s10552-008-9203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makari-Judson G, Judson CH, Mertens WC (2007) Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J 13 (3):258–265. doi:TBJ419 [pii] 10.1111/j.1524-4741.2007.00419.x [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Lu W, Gu K, Chen Z, Zheng Y, Zheng W, Shu XO (2011) Weight change and its correlates among breast cancer survivors. Nutr Cancer 63 (4):538–548. doi: 10.1080/01635581.2011.539316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu K, Chen X, Zheng Y, Chen Z, Zheng W, Lu W, Shu XO (2010) Weight change patterns among breast cancer survivors: results from the Shanghai breast cancer survival study. Cancer Causes Control 21 (4):621–629. doi: 10.1007/s10552-009-9491-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, Faerber S, Pierce JP (1999) Factors associated with weight gain in women after diagnosis of breast cancer. Journal of the American Dietetic Association 99 (10):1212–1221 [DOI] [PubMed] [Google Scholar]
- 8.Heideman WH, Russell NS, Gundy C, Rookus MA, Voskuil DW (2009) The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. Eur J Cancer 45 (1):119–126. doi: 10.1016/j.ejca.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Levine EG, Raczynski JM, Carpenter JT (1991) Weight gain with breast cancer adjuvant treatment. Cancer 67:1954–1959 [DOI] [PubMed] [Google Scholar]
- 10.DeGeorge D, Gray JJ, Fetting JH, Rolls BJ (1990) Weight gain in patients with breast cancer receiving adjuvant treatment as a function of restraint, disinhibition, and hunger. Oncology Nursing Forum 17 (3):23–30 [PubMed] [Google Scholar]
- 11.Loprinzi CL, Athmann LM, Kardinal CG, O’Fallon JR, See JA, Bruce BK, Dose AM, Miser AW, Kern PS, Tschetter LK, Rayson S (1996) Randomized trial of dietician counseling to try to prevent weight gain associated with breast cancer adjuvant chemotherapy. Oncology 53:228–232 [DOI] [PubMed] [Google Scholar]
- 12.Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH (1990) Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. Journal of Clinical Oncology 8 (8):1327–1334 [DOI] [PubMed] [Google Scholar]
- 13.Bonadonna G, Valagussa P, Rossi A, Tancini G, Brambilla C, Zambetti M, Veronesi U (1985) Ten-year experience with CMF-based adjuvant chemotherapy in resectable breast cancer. Breast cancer research and treatment 5:95–115 [DOI] [PubMed] [Google Scholar]
- 14.Heasman KZ, Sutherland HJ, Campbell JA, Elhakim T, Boyd NF (1985) Weight gain during adjuvant chemotherapy for breast cancer. Breast cancer research and treatment 5:195–200 [DOI] [PubMed] [Google Scholar]
- 15.Hoskin PJ, Ashley S, Yarnold JR (1992) Weight gain after primary surgery for breast cancer-effect of tamoxifen. Breast cancer research and treatment 22:129–132 [DOI] [PubMed] [Google Scholar]
- 16.Harvie MN, Campbell IT, Baildam A, Howell A (2004) Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast cancer research and treatment 83:201–210 [DOI] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Hars V, Conaway MR, Havlin K, Rimer BK, McElveen G, Winer EP (1997) Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy 1–3. American Journal of Clinical Nutrition 65:1495–1501 [DOI] [PubMed] [Google Scholar]
- 18.Cheney CL, Mahloch J, Freeny P (1997) Computerized tomography assessment of women with weight changes associated with adjuvant treatment for breast cancer 1–3. American Journal of Clinical Nutrition 66:141–146 [DOI] [PubMed] [Google Scholar]
- 19.Kumar N, Allen KA, Riccardi D, Bercu BB, Cantor A, Minton S, Balducci L, Jacobsen PB (2004) Fatigue, weight gain, lethargy and amenorrhea in breast cancer patients on chemotherapy: is subclinical hypothyroidism the culprit? Breast cancer research and treatment 83:149–159 [DOI] [PubMed] [Google Scholar]
- 20.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gillilad FD, Ballard-Barbash R (2005) Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. Journal of Clinical Oncology 23 (4):774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes JA, Knobf MT (2001) Weight gain and quality of life in women treated with adjuvant chemotherapy for early-stage breast cancer. Oncol Nurs Forum 28 (4):675–684 [PubMed] [Google Scholar]
- 22.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L (1996) Breast cancer survivors: Psychosocial concerns and quality of life. Breast cancer research and treatment 38:183–199 [DOI] [PubMed] [Google Scholar]
- 23.Herman DR, Ganz PA, Petersen L, Greendale GA (2005) Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast cancer research and treatment 93 (1):13–23 [DOI] [PubMed] [Google Scholar]
- 24.Imayama I, Alfano CM, Neuhouser ML, George SM, Wilder Smith A, Baumgartner RN, Baumgartner KB, Bernstein L, Wang CY, Duggan C, Ballard-Barbash R, McTiernan A (2013) Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast cancer research and treatment 140 (1):159–176. doi: 10.1007/s10549-013-2594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML (2015) Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. JNCI: Journal of the National Cancer Institute 107 (12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke CH, Chen WY, Rosner B, Holmes MD (2005) Weight, weight gain, and survival after breast cancer diagnosis. Journal of Clinical Oncology 23 (7):1370–1378 [DOI] [PubMed] [Google Scholar]
- 27.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W (2014) Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev 15 (9):749–768. doi: 10.1111/obr.12190 [DOI] [PubMed] [Google Scholar]
- 28.Thomson Z, Reeves M (2017) Can weight gain be prevented in women receiving treatment for breast cancer? A systematic review of intervention studies. Obesity Reviews 18 (11):1364–1373 [DOI] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine (2006) Guidelines for Exercise Testing and Prescription. 7th edn. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 30.Taves DR (1974) Minimization: a new method of assigning patients to treatment and control groups. Clinical Pharmacology & Therapeutics 15 (5):443–453 [DOI] [PubMed] [Google Scholar]
- 31.Parker PA, Youssef A, Walker S, Basen-Engquist K, Cohen L, Gritz ER, Wei QX, Robb GL (2007) Short-term and long-term psychosocial adjustment and quality of life in women undergoing different surgical procedures for breast cancer. Ann Surg Oncol 14 (11):3078–3089 [DOI] [PubMed] [Google Scholar]
- 32.Teo I, Novy DM, Chang DW, Cox MG, Fingeret MC (2015) Examining pain, body image, and depressive symptoms in patients with lymphedema secondary to breast cancer. Psychooncology. doi: 10.1002/pon.3745 [DOI] [PubMed] [Google Scholar]
- 33.DeSnyder SM, Teo I, Fingeret MC (2014) Body image struggles and breast cancer care: an under-recognized and undertreated issue with important implications for all patients. Breast Cancer Management 3 (3):251–260 [Google Scholar]
- 34.Bacon L, Stern JS, Van Loan MD, Keim NL (2005) Size acceptance and intuitive eating improve health for obese, female chronic dieters. Journal of the American Dietetic Association 105 (6):929–936 [DOI] [PubMed] [Google Scholar]
- 35.Khasteganan N, Lycett D, Furze G, Turner AP (2019) Health, not weight loss, focused programmes versus conventional weight loss programmes for cardiovascular risk factors: a systematic review and meta-analysis. Systematic reviews 8 (1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolls BJ, Bell EA (2000) Dietary approaches to the treatment of obesity. Medical Clinics of North America 84 (2):401–418 [DOI] [PubMed] [Google Scholar]
- 37.Bell S, Porter M, Kitchener H, Fraser C, Fisher P, Mann E (1995) Psychological response to cervical screening. Preventive Medicine 24:610–616 [DOI] [PubMed] [Google Scholar]
- 38.Drewnowski A, Almiron-Roig E, Marmonier C, Lluch A (2004) Dietary energy density and body weight: is there a relationship? Nutrition Reviews 62 (11):403–413 [DOI] [PubMed] [Google Scholar]
- 39.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T (2012) Nutrition and physical activity guidelines for cancer survivors. CA: a cancer journal for clinicians 62 (4):243–274. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 40.Rolls B (2005) The Volumetrics Eating Plan. 1st edn. HarperCollins, [Google Scholar]
- 41.Hopwood P, Fletcher I, Lee A, Ghazal SA (2001) A body image scale for use with cancer patients. European Journal of Cancer 37:189–197 [DOI] [PubMed] [Google Scholar]
- 42.Ware JE Jr, Snow KK, Kosinski M, Gandek B (1997) SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center, Boston, MA [Google Scholar]
- 43.Haberlin C, O’Dwyer T, Mockler D, Moran J, O’Donnell DM, Broderick J (2018) The use of eHealth to promote physical activity in cancer survivors: a systematic review. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 26 (10):3323–3336. doi: 10.1007/s00520-018-4305-z [DOI] [PubMed] [Google Scholar]
- 44.Lee JJ, Beretvas SN, Freeland-Graves JH (2014) Abdominal adiposity distribution in diabetic/prediabetic and nondiabetic populations: a meta-analysis. J Obes 2014:697264. doi: 10.1155/2014/697264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding JL, Shaw JE, Anstey KJ, Adams R, Balkau B, Brennan-Olsen SL, Briffa T, Davis TM, Davis WA, Dobson A, Flicker L, Giles G, Grant J, Huxley R, Knuiman M, Luszcz M, MacInnis RJ, Mitchell P, Pasco JA, Reid C, Simmons D, Simons L, Tonkin A, Woodward M, Peeters A, Magliano DJ (2015) Comparison of anthropometric measures as predictors of cancer incidence: A pooled collaborative analysis of 11 Australian cohorts. Int J Cancer. doi: 10.1002/ijc.29529 [DOI] [PubMed] [Google Scholar]
- 46.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Investigators IS (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364 (9438):937–952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 47.George SM, Bernstein L, Smith AW, Neuhouser ML, Baumgartner KB, Baumgartner RN, Ballard-Barbash R (2014) Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast cancer research and treatment 146 (3):647–655. doi: 10.1007/s10549-014-3048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL (2006) A self-regulation program for maintenance of weight loss. N Engl J Med 355 (15):1563–1571. doi: 10.1056/NEJMoa061883 [DOI] [PubMed] [Google Scholar]
- 49.Brown LF, Kroenke K, Theobald DE, Wu J (2011) Comparison of SF-36 vitality scale and Fatigue Symptom Inventory in assessing cancer-related fatigue. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19 (8):1255–1259. doi: 10.1007/s00520-011-1148-2 [DOI] [PubMed] [Google Scholar]
- 50.Reeves M, Winkler E, McCarthy N, Lawler S, Terranova C, Hayes S, Janda M, Demark-Wahnefried W, Eakin E (2017) The Living Well after Breast Cancer Pilot Trial: a weight loss intervention for women following treatment for breast cancer. Asia-Pacific journal of clinical oncology 13 (3):125–136. doi: 10.1111/ajco.12629 [DOI] [PubMed] [Google Scholar]
- 51.Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD (2010) Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv 4 (4):405–414. doi: 10.1007/s11764-010-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB (2010) Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 116 (24):5740–5748. doi: 10.1002/cncr.25294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, Springer BA, Pfalzer LA (2011) Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 19 (10):1581–1591. doi: 10.1007/s00520-010-0986-7 [DOI] [PMC free article] [PubMed] [Google Scholar]