Dear Editor,
Boucher-Neuhäuser syndrome (BNS) (MIM215470) has three key clinical features: cerebellar ataxia, hypogonadotropic hypogonadism, and chorioretinal dystrophy.1,2 BNS is inherited in an autosomal-recessive pattern and caused by mutations in PNPLA6 (MIM603197; 19p13.2).2,3 BNS is one of the disorders associated with PNPLA6 mutations that form part of a broad neurodegenerative spectrum with a similar phenotypic continuum.1,3,4
Here we report two siblings with typical phenotypes and PNPLA6 mutations of BNS.
A 30-year-old male (proband) and a 29-year-old female (sister of the proband) presented with chorioretinal dystrophy, cerebellar ataxia, and hypogonadotropic hypogonadism. The proband showed a delayed puberty and visual disturbance at the age of 12 years. He had no axillary or pubic hair, and a small penis and testis. Because of the hypogonadotropic hypogonadism, he had taken hormone therapy until the age of 26 years. He also showed mild dysarthria, dysphagia, gaze-evoked nystagmus, and peripheral neuropathy.
The sister of the proband presented with cerebellar ataxia and visual disturbances in her teens. She could not almost walk without assistance at the age of 28 years. She also showed dysarthria and alopecia. However, both siblings had never shown neurocognitive impairments. Serum testosterone of the proband was low. Follicle-stimulating hormone and luteinizing hormone were low in both siblings.
Electromyography of the proband produced normal findings, but a nerve conduction study showed no responses in the left common motor and superficial sensory peroneal nerves (Supplementary Fig. 1A in the online-only Data Supplement). Brain MRI of both siblings revealed prominent cerebellar atrophy, which was more severe in the proband (Supplementary Fig. 1B and C in the online-only Data Supplement).
In addition to the triad of clinical key features in BNS, ocular motor abnormalities, dysarthria, pyramidal tract signs, peripheral neuropathy, short stature, hair anomalies, and cognitive dysfunction have also commonly been reported.2,3,4 Both of the present siblings showed various clinical features in addition to the classical triad of features.
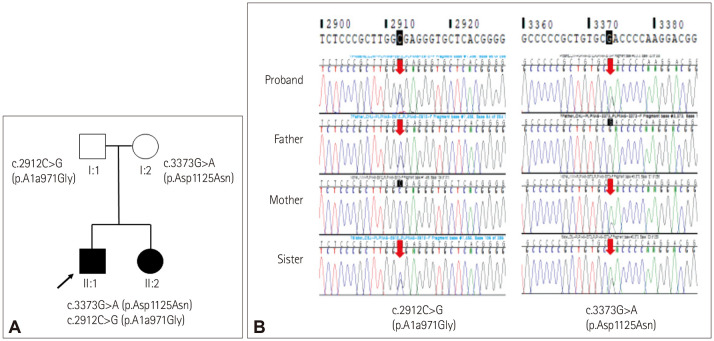
Clinical, laboratory, and electrophysiological findings are listed in Supplementary Table 1 (in the online-only Data Supplement). The two compound heterozygous mutations identified by whole-exome sequencing in both siblings comprised c.3373G>A (p.D1125N), which is a previously reported mutation, while c.2912C>G (p.A971G) is a novel mutation that has not been reported previously (Fig. 1). Compound heterozygous variants of both siblings were transmitted from the mutation carried by each parent. They are categorized as “likely pathogenic” variants by the American College of Medical Genetics guidelines.5
Fig. 1. Family pedigree and electropherograms of the PNPLA6 gene. A: The parents of the siblings each had one of the two PNPLA6 mutations. B: Compound heterozygous variants of the proband (II:1) and his sister (II:2) were inherited from both of their parents and caused their symptoms. The mother (I:2) had a missense heterozygous mutation, c.3373G>A (p.D1125N), in exon 29, and the father (I:1) had a missense heterozygous mutation, c.2912C>G (p.A971G), in exon 25.
Both compound heterozygous mutations of the two siblings were located in the phospholipid esterase domain (EST) of PNPLA6, which encodes neuropathy target esterase (NTE) that participates in phosphatidylcholine metabolism.4 Most PNPLA6 mutations are located in the EST, which affects the esterase activity of NTE, and PNPLA6 expression has been noted in the central nervous system and retina.4 Different impairments of NTE activity in PNPLA6, rather than the location of pathogenic variants, result in different clinical combinations.3,4,6 A greater reduction of NTE activity leads to more-severe and early-onset phenotypes.3,4 The clinical features and course of the same mutation different both between and within families.3,4,6 Therefore, it is necessary to continuously evaluate the phenotype–genotype correlation in BNS.
Footnotes
Ethics Statement: This work was approved by the institutional review board of Inje University Busan Paik Hospital (IRB No. BPIRB 2021-04-037-002). Written informed consent was obtained from both siblings and their parents before performing gene analyses.
- Conceptualization: Eun Joo Chung, Sang Jin Kim.
- Data curation: Seung Hwan Oh, Yun Joong Kim.
- Formal analysis: Woo Yeong Chung.
- Methodology: Eunkyoung You.
- Software: Go Hun Seo.
- Writing—original draft: Eun Joo Chung.
- Writing—review & editing: Eunkyoung You, Seung Hwan Oh, Go Hun Seo, Woo Yeong Chung, Yun Joong Kim, Sang Jin Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Supplementary Materials
The online-only Data Supplement is available with this article at at https://doi.org/10.3988/jcn.2022.18.2.233.
Electrophysiological findings of proband and comparison of brain MRI in both siblings. Findings of a nerve conduction study (NCS) and electromyography (EMG) of the proband (A). The NCS showed no responses in the left common peroneal nerve or the left superficial peroneal nerve. The EMG findings were normal. Brain MRI findings for the proband (B) and his sister (C). Axial (left) and sagittal (right) T1-weighted images of both siblings demonstrated diffuse cerebellar atrophy, but atrophy of the cerebral cortex and brainstem was not observed.
Clinical features of the patients
References
- 1.Zheng R, Zhao Y, Wu J, Wang Y, Liu JL, Zhou ZL, et al. A novel PNPLA6 compound heterozygous mutation identified in a Chinese patient with Boucher-Neuhäuser syndrome. Mol Med Rep. 2018;18:261–267. doi: 10.3892/mmr.2018.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarnutzer AA, Gerth-Kahlert C, Timmann D, Chang DI, Harmuth F, Bauer P, et al. Boucher-Neuhäuser syndrome: cerebellar degeneration, chorioretinal dystrophy and hypogonadotropic hypogonadism: two novel cases and a review of 40 cases from the literature. J Neurol. 2015;262:194–202. doi: 10.1007/s00415-014-7555-9. [DOI] [PubMed] [Google Scholar]
- 3.Synofzik M, Gonzalez MA, Lourenco CM, Coutelier M, Haack TB, Rebelo A, et al. PNPLA6 mutations cause Boucher-Neuhauser and Gordon Holmes syndromes as part of a broad neurodegenerative spectrum. Brain. 2014;137:69–77. doi: 10.1093/brain/awt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hufnagel RB, Arno G, Hein ND, Hersheson J, Prasad M, Anderson Y, et al. Neuropathy target esterase impairments cause Oliver-McFarlane and Laurence-Moon syndromes. J Med Genet. 2015;52:85–94. doi: 10.1136/jmedgenet-2014-102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo GH, Kim T, Choi IH, Park JY, Lee J, Kim S, et al. Diagnostic yield and clinical utility of whole exome sequencing using an automated variant prioritization system, EVIDENCE. Clin Genet. 2020;98:562–570. doi: 10.1111/cge.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kmoch S, Majewski J, Ramamurthy V, Cao S, Fahiminiya S, Ren H, et al. Mutations in PNPLA6 are linked to photoreceptor degeneration and various forms of childhood blindness. Nat Commun. 2015;6:5614. doi: 10.1038/ncomms6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electrophysiological findings of proband and comparison of brain MRI in both siblings. Findings of a nerve conduction study (NCS) and electromyography (EMG) of the proband (A). The NCS showed no responses in the left common peroneal nerve or the left superficial peroneal nerve. The EMG findings were normal. Brain MRI findings for the proband (B) and his sister (C). Axial (left) and sagittal (right) T1-weighted images of both siblings demonstrated diffuse cerebellar atrophy, but atrophy of the cerebral cortex and brainstem was not observed.
Clinical features of the patients
Data Availability Statement
All data generated or analyzed during the study are included in this published article (and its supplementary information files).