FIGURE 2.
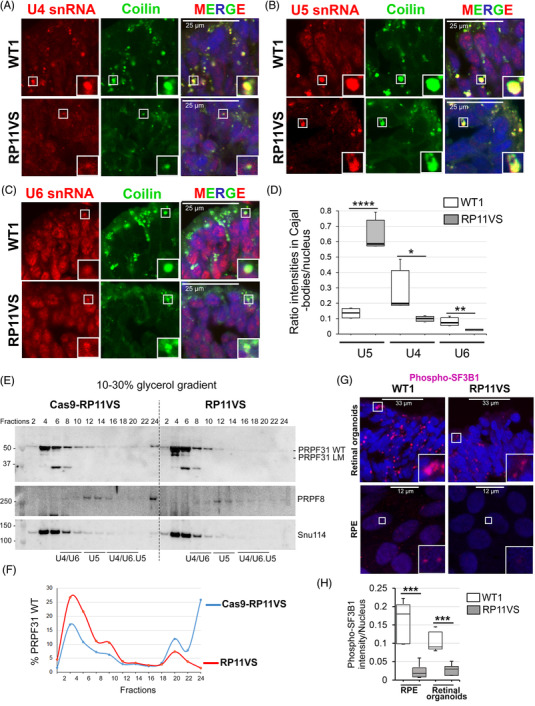
PRPF31 RP mutations lead to defects in the assembly of tri‐snRNPs in Cajal bodies, and reduce formation of active spliceosomes (Bact, C complexes) in RPE and photoreceptor cells. (A–C) Confocal microscopy analyses of RNA‐FISH labelling for U4 (A), U5 (B), and U6 (C) snRNAs (red) in Cajal bodies (anti‐coilin, green) in control and RP11VS retinal organoids. (D) Quantification of the mean intensity ratios of accumulation of snRNAs in Cajal bodies in RP11VS and control RPE cells. PRPF31 RP mutations lead to the significant accumulation of U5 snRNA and reduction of U4/U6 snRNAs in Cajal bodies. A total of 200–300 cells per experiment and at least two independent experiments were measured and plotted. The significance was analysed by t‐test; *P < 0.05, **P < 0.01, ****P < 0.0001. (E) Western blots of even‐numbered glycerol gradient fractions of isogenic control and RP11VS‐RPE cells showing accumulation of WT PRPF31 and long mutant (LM) in the top fractions in RP11VS RPE cells. (F) Quantification of the percentage of WT PRPF31 in gradient fractions in Cas9‐RP11VS and RP11VS‐RPE cells. (G) Staining of retinal organoids and RPE cells with anti‐phosphorylated SF3B1 antibodies showing reduced levels of active spliceosomes (Bact and C complexes) in both RP11VS RPE and photoreceptor cells. (H) Quantification of the mean intensity of phospho‐SF3B1 in RP11‐RPE/retinal and control cells. A total of 200–300 cells per experiment and at least two independent experiments were measured and plotted. The significance was analysed by t‐test; ***P < 0.001.
