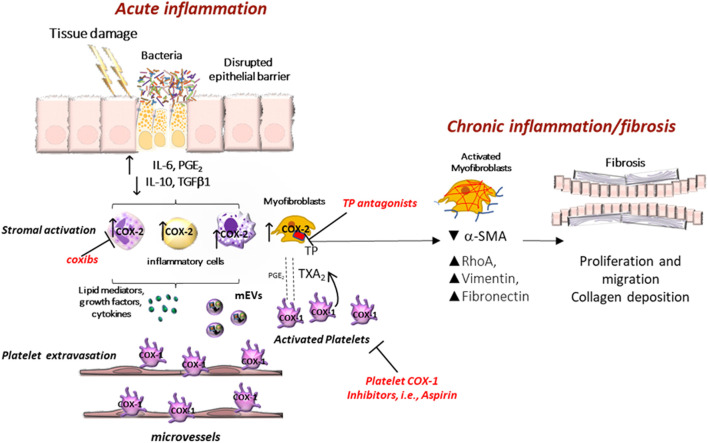
FIGURE 2.
Clinical implications of eicosanoid inhibition in intestinal tumorigenesis. Activated platelets release soluble mediators, including thromboxane A2 (TXA2), prostaglandin E2 (PGE2), 12S-hydroxyeicosatetraenoic acid (12S-HETE), growth factors, cytokines, and platelet-derived medium-sized extracellular vesicles (mEVs) containing microRNAs, which contribute to the phenotypic changes of the cells in the stromal microenvironment. Interactions between epithelial cells and stromal cells undergo an alteration that induces the development of intestinal neoplasia. A crucial event is the enhanced biosynthesis of PGE2 in the intestinal mucosa, occurring in the early stages of tumor development via the cyclooxygenase (COX)-1 activity, in association with the suppression of the prostaglandin-degrading enzyme 15-prostaglandin dehydrogenase (15-PGDH). Later, the overexpression of COX-2 contributes to the generation of aberrant levels of PGE2 in the stromal compartment and epithelial cells. PGE2 plays multifaceted roles in cancer promotion, including proliferation, migration, epithelial-mesenchymal transition (EMT), and immune escape. Tumor cells that undergo this phenomenon acquire a migratory capacity that facilitates metastatic colonization. By inhibiting platelet activation, antiplatelet agents can indirectly restrain the induction of COX-2 in target cells. Differently, selective COX-2 inhibitors (coxibs) affect COX-2 activity already expressed in the tumor microenvironment and cancer cells. 12S-HETE generated by 12-lipoxygenase (LOX) may have protumorigenic effects, and selective 12-LOX inhibitors are in clinical development.