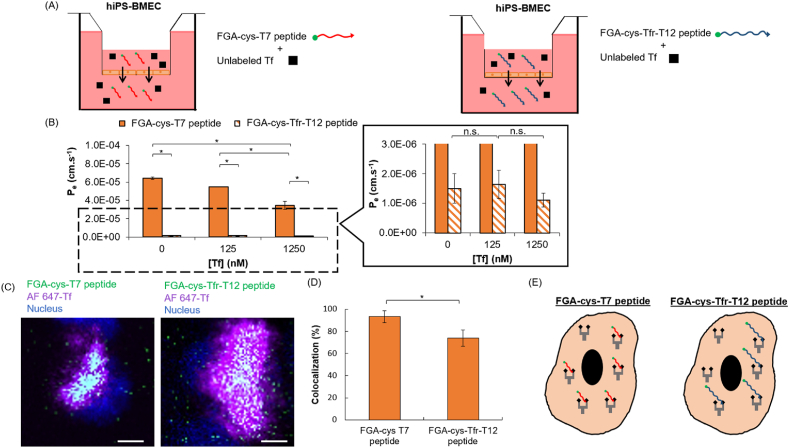
Fig. 7.
Competitive binding assay between 10 μg/mL of FGA-cys-T7 and FGA-cys-Tfr-T12 peptide and various concentrations (0; 125; 1250 nM) of unlabeled Tf with hiPS-BMEC (n = 3). (A) Schematic illustration of the method. The medium in the bottom compartment was collected at determined time to measure fluorescence intensity. (B) Effective permeability coefficient of 10 μg/mL FGA-cys-T7 peptide (full bars) and FGA-cys-Tfr-T12 peptide (hatched bars) when co-incubated with various concentrations of unlabeled Tf by hiPS-BMEC after 1 h incubation at 37 °C. Data are presented as means ± S.D. Statistical analysis was performed using one-way ANOVA (∗p ≤ 0.05, n. s. p > 0.05). The biological replicates of hiPS-BMEC are issued from the same differentiation. (C) Colocalization of FGA-cys-T7 or FGA-cys-Tfr-T12 peptide and Alexa Fluor 647- transferrin (AF 647-Tf) with hiPS-BMEC. Scale bar = 10 μm. The biological replicates of hiPS-BMEC are issued from the same differentiation. (D) Quantification of the colocalization percentage between FGA-peptides and AF 647-Tf using hiPS-BMEC calculated with IMARIS software. Data are presented as means ± S.D. Statistical analysis was performed using the Student's t-test (∗p ≤ 0.05). (E) Schematic illustration of the interaction between FGA-cys-T7 or FGA-cys-Tfr-T12 peptide and Tf for the binding to TfR with hiPS-BMEC.