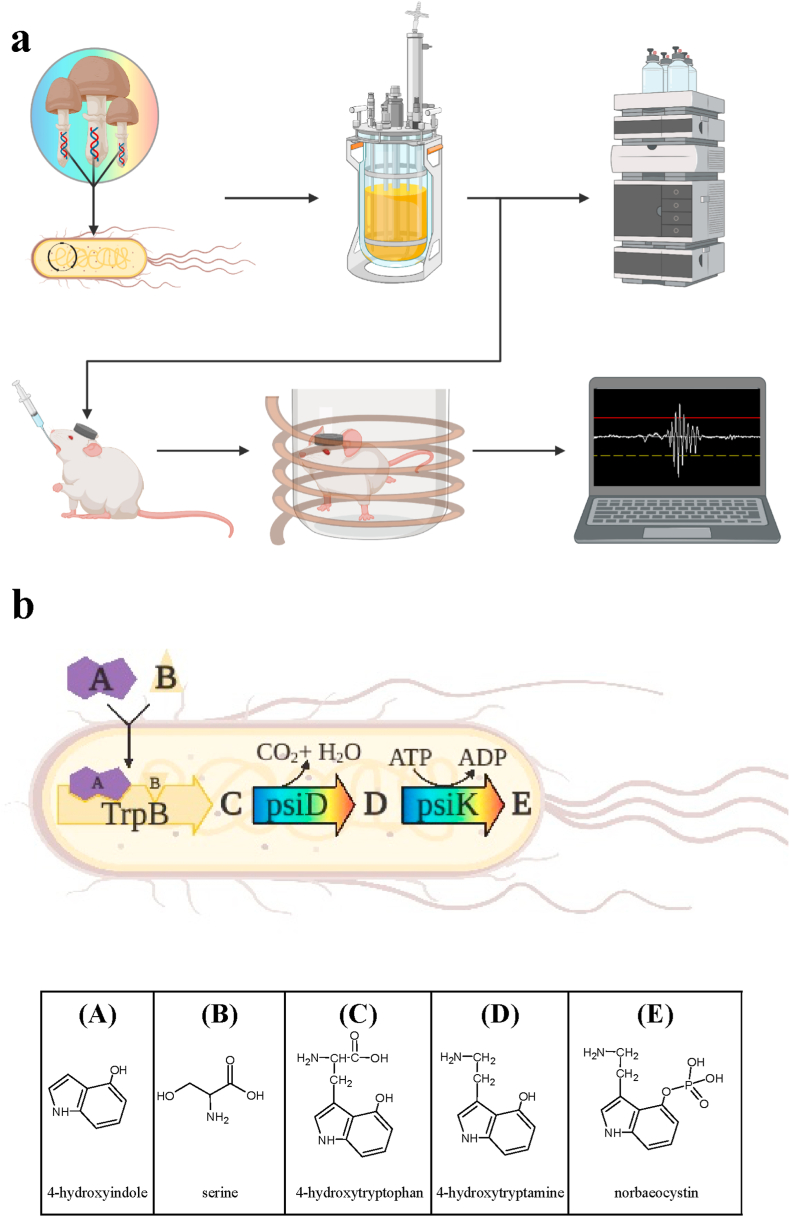
Fig. 1.
(a) Overview of study methods. Recombinant E. coli were developed capable of high-level norbaeocystin production. Norbaeocystin production was optimized and scaled up in a benchtop bioreactor. Norbaeocystin concentration in cell broth was quantified using HPLC. A rat with a magnet affixed to its head was gavaged with psilocybin or norbaeocystin in either a cell broth or water vehicle. A magnetometer coil was used in order to record head twitches. Waveforms were then analyzed to determine the head twitch count. (b) Norbaeocystin biosynthesis pathway. The E. coli strain contains three genes, one native (trpB) and two heterologous (psiD, psiK) that enable norbaeocystin biosynthesis from external supplementation of 4-hydroxyindole. Tryptophan synthase (TrpB) condenses 4-hydroxyindole and serine to form 4-hydroxytryptophan. P. cubensis tryptophan decarboxylase (PsiD) converts 4-hydroxytryptophan into 4-hydroxytryptamine while releasing a carbon dioxide and water. Finally, P. cubensis kinase (PsiK) converts 4-hydroxytryptamine into norbaeocystin using a phosphate donated by ATP.