FIGURE 3.
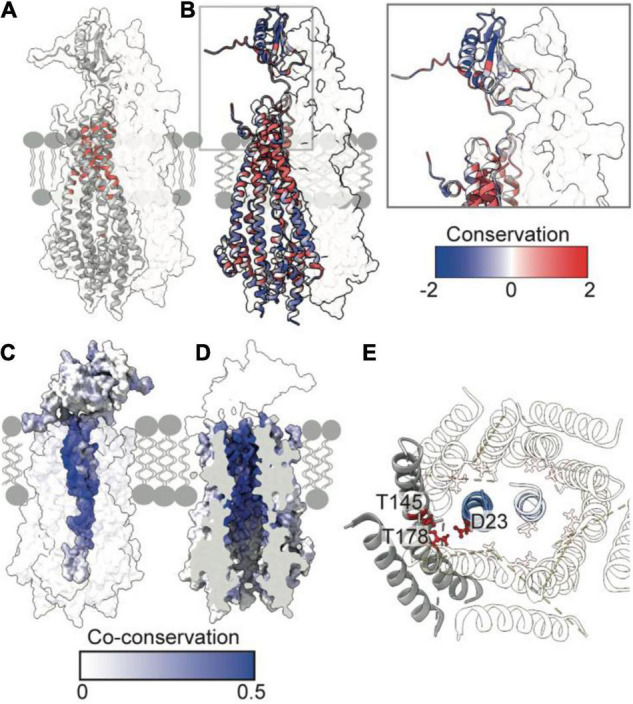
Essential, conserved, and co-conserved stator residues map to the TolQR lumen. (A) Red identifies essential residues that confer membrane instability when substituted (Vianney et al., 1994; Goemaere et al., 2007; Zhang et al., 2011). Two TolQ monomers are shown in cartoon format with a single TolR monomer. (B) The relative conservation of TolQ-TolR residues, which was generated following alignment (Clustal Omega) of ten TolQ sequences from different species, indicates that the most conserved residues map to the top or bottom of the TolQ lumen. The most-conserved residues of TolR map to the transpore helix and β-strand two. (C,D) The TolQ-TolR co-conservation profile of individual residues as scored by RaptorX suggest that the TolR helix inserts into the center of the TolQ pore. Co-conserved TolR and TolQ residues are predominantly within the transpore helix and lumen, respectively. (E) A ring of highly conserved threonine residues (red sticks) within the transmembrane region of TolQ (T145 in helix 6 and T178 in helix 7) are proposed to stabilize protonated TolR D23 (red sticks), based on structures of the MotA-MotB and ExbB-ExbD complexes (Celia et al., 2019; Deme et al., 2020).
