FIGURE 4.
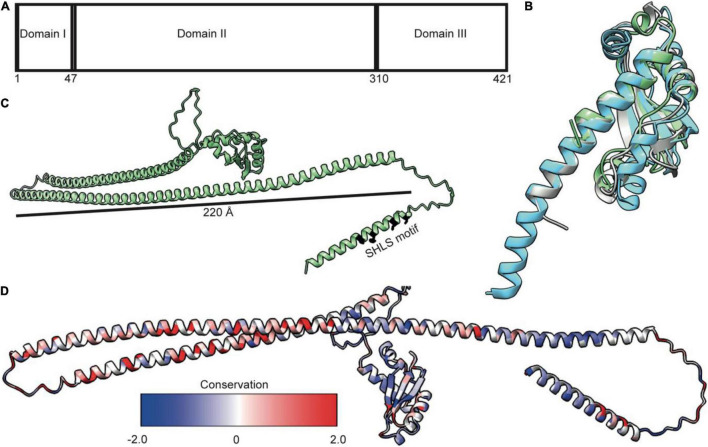
TolA is a largely helical protein with three distinct domains. (A) TolA contains three annotated domains: domain I is the transmembrane helix, domain II is an elongated helical hairpin, and domain III is a small globular protein. Numbering is based on the E. coli sequence. (B) Structural overlay of TolA III from P. aeruginosa (cyan, PDB ID 1LR0) and V. cholerae (gray, PDB ID 4G7X) with the equivalent domain from E. coli (green, PDB ID 1S62) (RMSD of 1.3 and 2.0, respectively) showing that, although poorly conserved (20 and 25%, respectively), the domains have near identical folds. (C) The AlphaFold model of TolA from E. coli. The central domain II is predicted to form a helical hairpin that is 220 Å in length, which is long enough to span the periplasm. (D) Sequences of nine TolA proteins from different species were aligned in Clustal Omega, and sequence conservation was mapped onto the E. coli TolA structure within ChimeraX. Conserved residues are largely located within the C-terminal end of the helical hairpin of domain II. Residues in domains I and III are less conserved.