FIGURE 5.
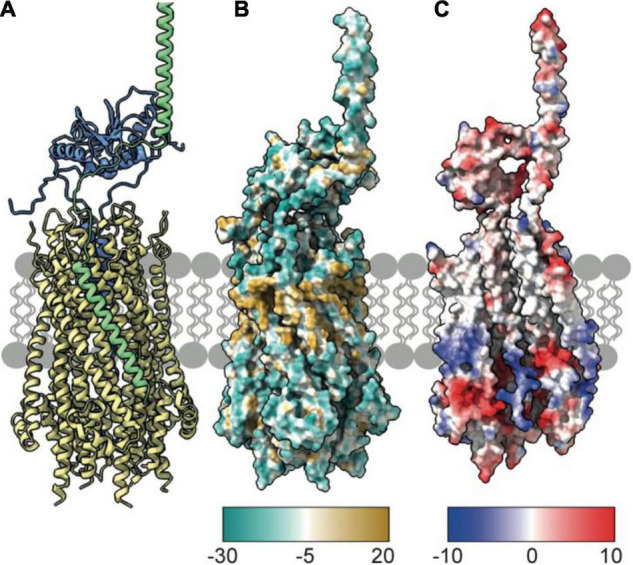
TolA packs against the TolQ pentamer to form the TolQRA complex. (A) The Rosetta model of the TolQ-TolR-TolA complex from E. coli was generated following placement of full length TolA, taken from the AlphaFold database, within 10 Å of the TolQ-TolR complex (Figure 2). A series of Rosetta docking, and refinement steps were run to generate the lowest energy model shown. TolA (green) packs against the TolQ pentamer (yellow) with the transmembrane helix of TolA placed in a parallel arrangement within the exterior helices of TolQ. The TolR dimer sits above TolQ with its transpore helices packed within the TolQ pore. The model suggests that TolR is in close proximity to the hairpin (domain II) of TolA. (B) Hydrophobicity map of the TolQ-TolR-TolA complex shows a hydrophobic (gold) belt around the middle of the TolQ-TolA complex. This depiction is consistent with this region residing in the IM. (C) Electrostatic properties of the TolQ-TolR-TolA complex are displayed with chains B and C from TolQ hidden to show the charge within the pore. The extreme N-terminus of TolR is electropositive, complementing the electronegative charge within the pore. A similar charge-coupling is seen between the transmembrane helix of TolA and the exterior of TolQ. The TolQ-TolR-TolA model is available as a PDB file from associated Supplementary Material.
