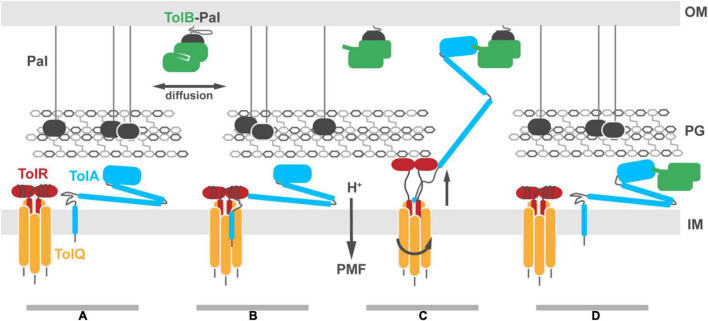
FIGURE 7.
Putative model for Tol-Pal force transduction through the cell envelope. (A) The TolQ-TolR stator and TolA diffuse freely within the IM. The abundant OM lipoprotein Pal is predominantly bound to the PG, stabilizing its connection to the OM. A sub-population of Pal molecules are bound to TolB. This binding blocks association of those Pal molecules with PG, enhancing their diffusion in the OM. (B) TolA associates with the TolQ-TolR stator, inserting between monomers of TolQ. (C) In response to protonation of stator residues, TolR extends and binds the PG layer, allowing rotation of the TolQ helices, which in turn drives extension of the TolA helical hairpin through pores in the PG layer. The C-terminal domain of TolA associates with the N-terminus of TolB in its complex with Pal. (D) Deprotonation of stator residues drive retraction of the TolR periplasmic domain away from the PG. Relaxation of the TolA helical hairpin toward the IM could be either through coupling to this structural change or refolding of the hairpin, following dissociation of TolA from the stator (as shown in the panel). We speculate that retraction of the TolA hairpin provides the driving force for dissociating TolB-Pal complexes at the OM and pulling TolB below the PG layer. The figure does not show recruitment of the TolQ-TolR-TolA complex to the divisome which concentrates Pal deposition at division sites to stabilize the invaginating OM.