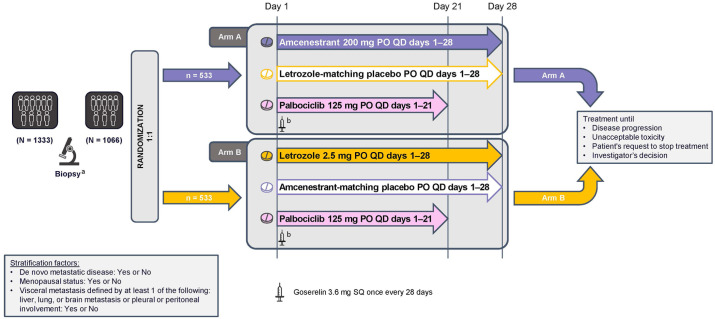
Figure 2.
AMEERA-5 study design. Randomization will be stratified by de novo metastatic disease (yes or no), menopausal status (yes or no), and visceral metastasis defined by at least one of the following: liver, lung, or brain metastasis or pleural or peritoneal involvement (yes or no).
PO, oral; QD, once daily; SQ, subcutaneous.
aArchived tissue or fresh sample obtained between screening and cycle 1 day 1.
bPre-/perimenopausal women and men will receive a subcutaneous goserelin implant (3.6 mg) on day 1 of every 28-day cycle.