Figure 3.
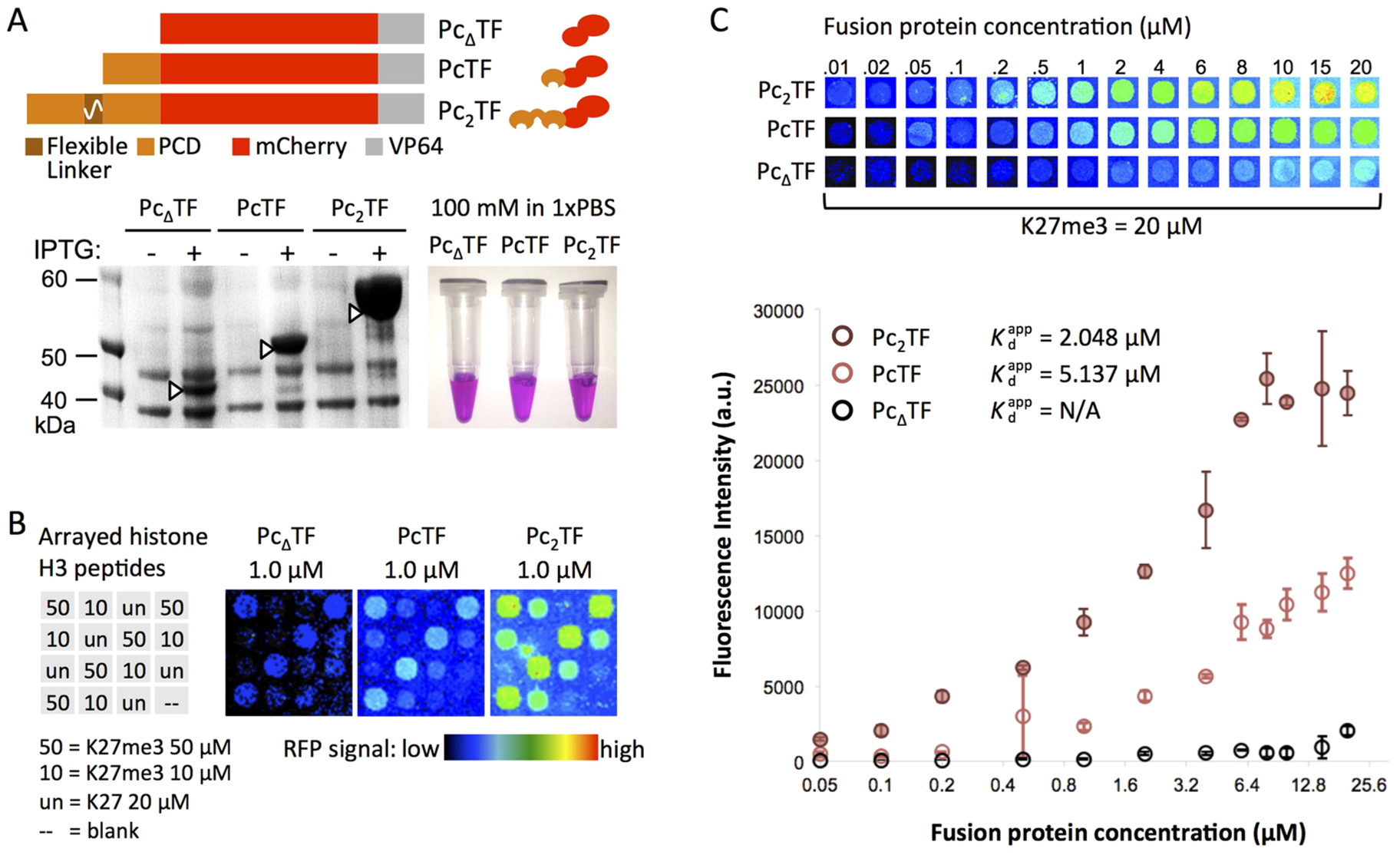
A bivalent PCD fusion peptide shows enhanced avidity for H3K27me3 in microspot array experiments. (A) For high-yield expression, E. coli was transformed with pET28 plasmids encoding PcΔTF (negative control), PcTF (single PCD), and the Pc2TF containing the flexible linker (GGGGS)4. Native polyacrylamide gel electrophoresis (PAGE) of overexpressed proteins purified from E. coli. (B) Test slides were spotted with histone H3 peptides (K27me3 or unmodified K27) as indicated in the grid for qualitative analysis. Pseudocolored images show mCherry signal after an application of 1.0 μM fusion protein to individual arrays. (C) New arrays were spotted with 10, 20, and 50 μM H3K27me3 for quantitative analysis. Fluorescence signal versus the concentration of fusion protein applied to the array was used to calculate the apparent dissociation constant (, not applicable for PcΔTF). Each point in the graph is the mean signal from four spots in one application (error bars = SDM). The data displayed in the graph are from representative applications (out of four total) for 20 μM immobilized H3K27me3.
