Figure 1. Discovering a FEM1B recruiter.
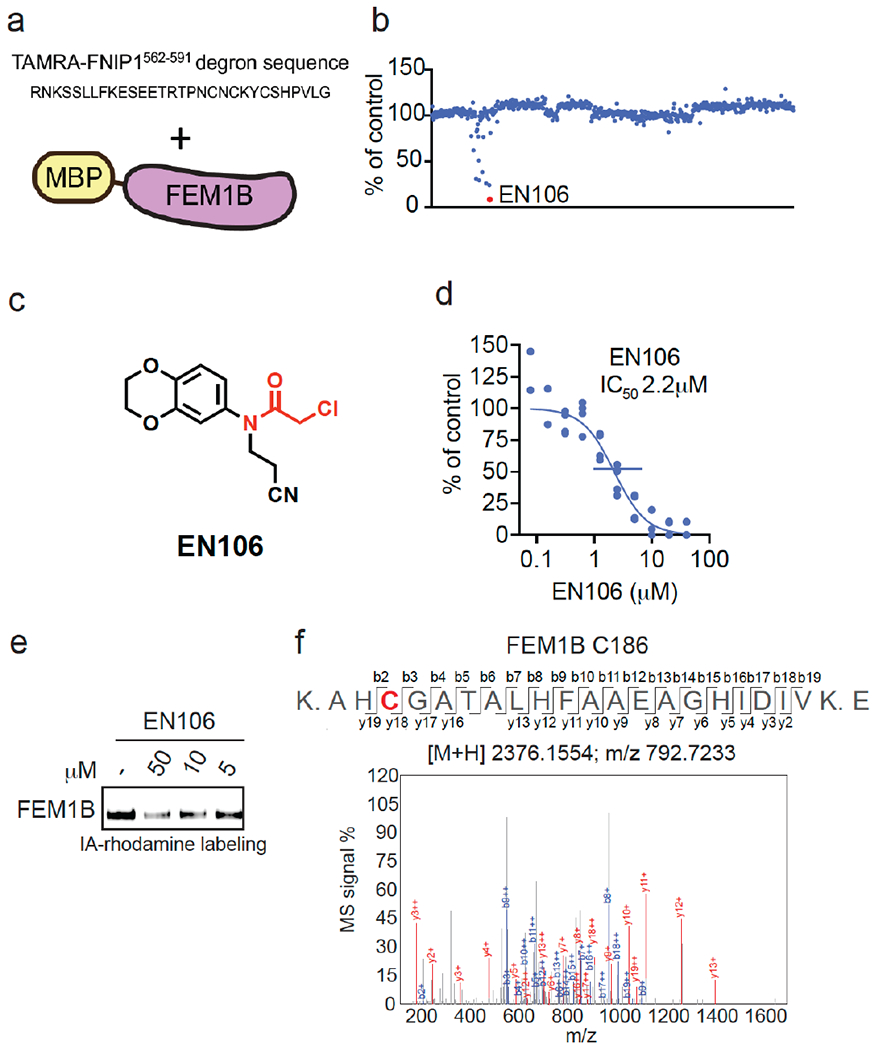
(a, b) Screening a cysteine-reactive covalent ligand library in a fluorescence polarization assay with TAMRA-conjugated FNIP1562-591 degron with recombinant MBP-tagged FEM1B (a). FEM1B was pre-incubated with DMSO vehicle or covalent ligand (50 μM) for 1 h prior to addition of the TAMRA-conjugated degron (b). Data is in Table S1. (c) Structure of EN106 with covalent chloroacetamide handle in red. (d) Dose-response of EN106 inhibition of FEM1B and TAMRA-conjugated FNIP1 interaction assessed by fluorescence polarization expressed as percent fluorescence polarization compared to DMSO control. (e) Gel-based ABPP of EN106. 50 nM pure FEM1B protein was pre-treated with DMSO or EN106 for 30 min at room temperature prior to addition of IA-rhodamine (500 nM, 30 min) at room temperature, after which protein was resolved on SDS/PAGE and visualized by in-gel fluorescence. (f) Site of modification of EN106 on FEM1B. FEM1B was labeled with EN106 (50 μM) for 30 min, and FEM1B tryptic digests were analyzed by LC-MS/MS for the EN106 adduct. Data in (b) show average from n=2/group. Data in (d) shows individual data replicates from n=2-4/group. Data in (e) shows representative gel from n=3/group.
