Table 2. Experimental and Fitted Solubility Data of MET·HCl in Water + Acetonitrile (P = 0.1 MPa)a,b.
| 103x1exp | 103x1apelblat | 103x1CNIBS | 103x1AJA | 103x1λh | ||
|---|---|---|---|---|---|---|
| 283.15 K | ||||||
| 0.30 | 3.730 | 3.656 | 3.825 | 3.394 | 3.648 | |
| 0.40 | 7.404 | 7.356 | 6.648 | 7.137 | 7.154 | |
| 0.50 | 9.214 | 9.898 | 10.84 | 11.94 | 11.07 | |
| 0.60 | 17.61 | 17.43 | 16.26 | 17.09 | 17.16 | |
| 0.70 | 22.86 | 22.85 | 22.14 | 21.99 | 23.05 | |
| 0.80 | 26.14 | 25.62 | 27.20 | 26.20 | 25.76 | |
| 0.90 | 30.29 | 29.75 | 30.16 | 29.15 | 30.92 | |
| 1.00 | 30.45 | 30.27 | 30.36 | 29.89 | 31.26 | |
| 288.15 K | ||||||
| 0.30 | 3.861 | 4.016 | 3.872 | 3.823 | 4.019 | |
| 0.40 | 8.137 | 8.103 | 8.035 | 7.995 | 8.019 | |
| 0.50 | 13.15 | 11.94 | 13.38 | 13.36 | 12.54 | |
| 0.60 | 18.93 | 19.27 | 19.06 | 19.14 | 19.16 | |
| 0.70 | 25.49 | 25.49 | 24.40 | 24.70 | 25.58 | |
| 0.80 | 27.56 | 28.58 | 28.96 | 29.56 | 28.67 | |
| 0.90 | 33.17 | 34.04 | 32.37 | 33.07 | 34.72 | |
| 1.00 | 33.85 | 34.32 | 34.01 | 34.15 | 34.62 | |
| 293.15 K | ||||||
| 0.30 | 4.530 | 4.407 | 4.504 | 4.292 | 4.418 | |
| 0.40 | 8.710 | 8.945 | 8.875 | 8.922 | 8.963 | |
| 0.50 | 14.95 | 14.11 | 14.73 | 14.88 | 14.15 | |
| 0.60 | 21.39 | 21.30 | 21.35 | 21.32 | 21.33 | |
| 0.70 | 28.21 | 28.31 | 27.85 | 27.58 | 28.30 | |
| 0.80 | 32.03 | 31.78 | 33.39 | 33.13 | 31.81 | |
| 0.90 | 38.59 | 38.50 | 37.31 | 37.24 | 38.45 | |
| 1.00 | 38.75 | 38.66 | 39.09 | 38.69 | 38.63 | |
| 298.15 K | ||||||
| 0.30 | 4.688 | 4.831 | 4.687 | 4.802 | 4.847 | |
| 0.40 | 9.941 | 9.894 | 9.920 | 9.919 | 9.989 | |
| 0.50 | 16.43 | 16.34 | 16.55 | 16.50 | 15.91 | |
| 0.60 | 23.62 | 23.54 | 23.65 | 23.64 | 23.67 | |
| 0.70 | 31.50 | 31.33 | 30.64 | 30.63 | 31.24 | |
| 0.80 | 35.61 | 35.21 | 37.09 | 36.89 | 35.19 | |
| 0.90 | 43.16 | 43.10 | 42.12 | 41.63 | 42.46 | |
| 1.00 | 43.60 | 43.25 | 43.83 | 43.49 | 42.97 | |
| 303.15 K | ||||||
| 0.30 | 5.517 | 5.290 | 5.510 | 5.355 | 5.308 | |
| 0.40 | 11.11 | 10.96 | 11.11 | 10.99 | 11.10 | |
| 0.50 | 18.07 | 18.57 | 18.18 | 18.22 | 17.84 | |
| 0.60 | 25.98 | 26.02 | 25.88 | 26.09 | 26.21 | |
| 0.70 | 34.43 | 34.53 | 33.60 | 33.84 | 34.40 | |
| 0.80 | 39.06 | 38.89 | 40.81 | 40.85 | 38.84 | |
| 0.90 | 47.83 | 47.76 | 46.48 | 46.25 | 46.77 | |
| 1.00 | 48.33 | 48.09 | 48.65 | 48.53 | 47.66 | |
| 308.15 K | ||||||
| 0.30 | 5.834 | 5.787 | 5.833 | 5.956 | 5.804 | |
| 0.40 | 12.28 | 12.17 | 12.24 | 12.13 | 12.31 | |
| 0.50 | 19.97 | 20.73 | 20.23 | 20.05 | 19.95 | |
| 0.60 | 28.88 | 28.76 | 28.66 | 28.67 | 28.95 | |
| 0.70 | 37.81 | 37.94 | 36.93 | 37.20 | 37.79 | |
| 0.80 | 42.75 | 42.83 | 44.66 | 44.97 | 42.76 | |
| 0.90 | 52.37 | 52.43 | 50.93 | 51.04 | 51.39 | |
| 1.00 | 53.25 | 53.17 | 53.59 | 53.77 | 52.71 | |
| 313.15 K | ||||||
| 0.30 | 6.261 | 6.324 | 6.257 | 6.604 | 6.336 | |
| 0.40 | 13.43 | 13.53 | 13.42 | 13.36 | 13.62 | |
| 0.50 | 22.10 | 22.77 | 22.30 | 21.97 | 22.24 | |
| 0.60 | 31.74 | 31.78 | 31.60 | 31.38 | 31.90 | |
| 0.70 | 41.75 | 41.54 | 40.73 | 40.70 | 41.44 | |
| 0.80 | 47.20 | 47.03 | 49.31 | 49.25 | 46.97 | |
| 0.90 | 57.67 | 57.05 | 56.09 | 56.01 | 56.35 | |
| 1.00 | 57.72 | 58.46 | 58.09 | 59.18 | 58.15 | |
| 318.15 K | ||||||
| 0.30 | 6.739 | 6.90 | 6.742 | 7.303 | 6.908 | |
| 0.40 | 15.03 | 15.06 | 14.96 | 14.66 | 15.05 | |
| 0.50 | 24.68 | 24.62 | 24.99 | 24.01 | 24.73 | |
| 0.60 | 35.20 | 35.11 | 35.00 | 34.20 | 35.09 | |
| 0.70 | 45.31 | 45.33 | 44.31 | 44.33 | 45.34 | |
| 0.80 | 51.00 | 51.50 | 52.94 | 53.66 | 51.49 | |
| 0.90 | 61.76 | 61.56 | 60.36 | 61.11 | 61.65 | |
| 1.00 | 63.98 | 63.94 | 64.31 | 64.73 | 63.98 | |
| 323.15 K | ||||||
| 0.30 | 7.65 | 7.531 | 7.669 | 8.056 | 7.522 | |
| 0.40 | 16.77 | 16.78 | 16.60 | 16.04 | 16.58 | |
| 0.50 | 27.20 | 26.23 | 27.60 | 26.14 | 27.44 | |
| 0.60 | 38.67 | 38.78 | 38.59 | 37.14 | 38.51 | |
| 0.70 | 49.28 | 49.33 | 48.55 | 48.08 | 49.53 | |
| 0.80 | 56.44 | 56.24 | 57.37 | 58.19 | 56.34 | |
| 0.90 | 65.34 | 65.90 | 64.90 | 66.32 | 67.32 | |
| 1.00 | 69.87 | 69.59 | 69.95 | 70.39 | 70.25 | |
x1 is
the experimental solubility; the x1apelblat, x1CNIBSx1λh, and x1AJA are the calculated mole solubilities
according to the modified Apelblat model, CNIBS/R-K model, λh model, and Apelblat–Jouyban–Acree model,
respectively. 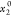 represents the initial mole fraction of
water in the binary aqueous solvent.
represents the initial mole fraction of
water in the binary aqueous solvent.
The standard uncertainty is u(T) = 0.1 K; the relative standard uncertainties ur are ur(P) = 0.01, ur(x1) = 0.05, and ur(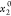 ) = 0.001.
) = 0.001.
