Figure 7.
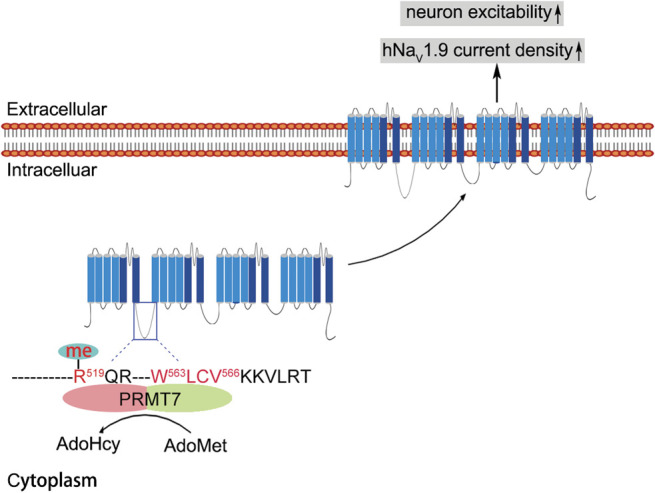
Proposed working model of PRMT7-mediated NaV1.9 trafficking and cellular excitability. The PRMT7 C-terminal domain interacts with residues 563 to 566 of hLoop1 and methylates arginine 519 (R519 me) in this loop using S-adenosyl-L-methionine (AdoMet) as a methyl donor to produce S-adenosylhomocysteine (AdoHcy). Human NaV1.9; R519 me is involved in the regulation of NaV1.9 trafficking to the cell surface through an undefined mechanism. Consequently, altered cell surface expression of NaV1.9 increases sodium current density, leading to hyperexcitability of DRG neurons. DRG, dorsal root ganglion; PRMT7, protein arginine methyltransferase 7.
