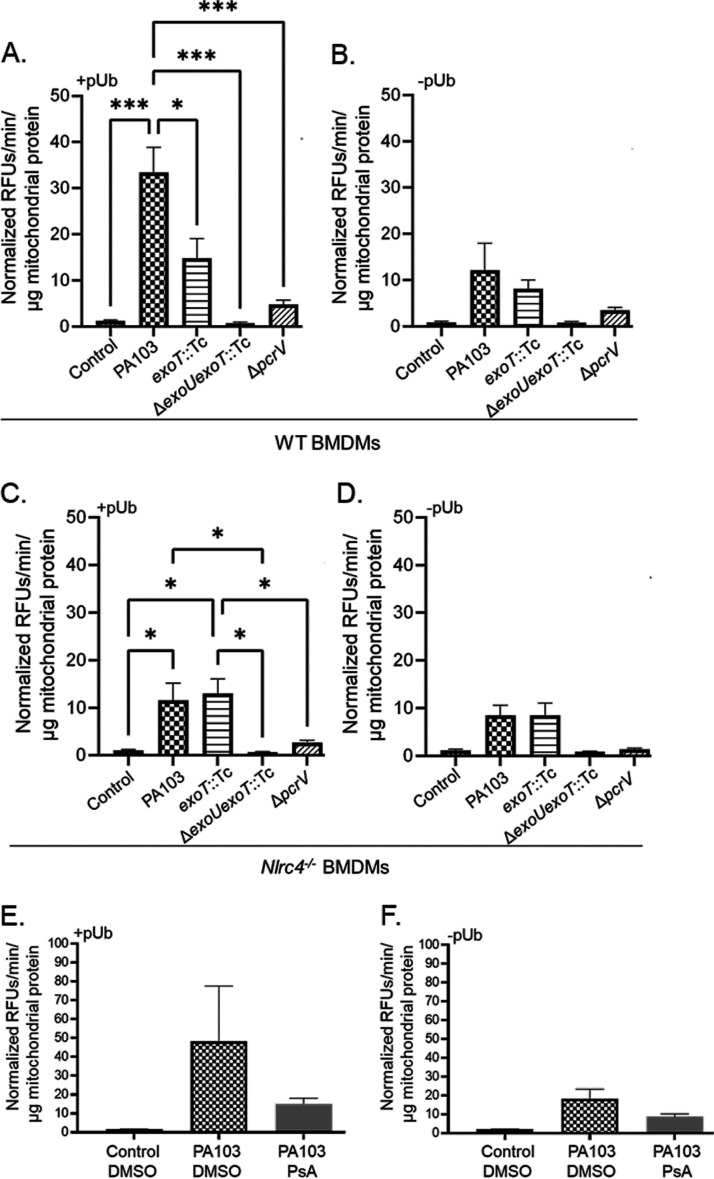
FIG 3.
ExoU associates with mitochondria via NLRC4 and ExoT. (A and B) WT BMDMs were treated with sterile saline (control) or inoculated with P. aeruginosa strain PA103, PA103 exoT::Tc, PA103 ΔexoU exoT::Tc, or PA103 ΔpcrV at an MOI of 40:1. At 180 min postinoculation, enriched mito-MAMs were isolated and suspended directly into buffered solution containing the fluorogenic PLA2 substrate PED6. Samples were then split into two separate reactions. Panel A shows reactions where pUb was added (0.1 mg/mL; +pUb) to stimulate ExoU PLA2 activity in the enriched mito-MAM fractions. Panel B shows control reactions where pUb was not added (–pUb). All raw data (relative fluorescent units [RFUs]) were corrected for background fluorescence, and PLA2 activity is expressed as the rate of PED6 hydrolysis (in minutes), normalized to total mitochondrial protein in each reaction (in micrograms). (C and D) Nlrc4−/− BMDMs were treated and tested for ExoU using the PED6 PLA2 assay as described for panels A and B. Panel C shows reactions where pUb was added (0.1 mg/mL; +pUb), and panel D shows control reactions where pUb was not added (–pUb). ***, P value ≤ 0.0005; *, P value ≤ 0.04. (E and F) WT BMDMs were preincubated with DMSO (vehicle control) or the ExoU-specific inhibitor PsA (50 μM) for 60 min prior to inoculation. Subsequently, cultures were treated with sterile saline (control) or inoculated with P. aeruginosa strain PA103 for 180 min (DMSO or PsA was maintained throughout the time course). Enriched mito-MAM fractions were isolated and tested for ExoU using the PED6 PLA2 assay. Panel E shows reactions where pUb was added (0.1 mg/mL), and panel F shows control reactions where pUb was not added. Data in all panels are representative of 3 independent experiments.