Abstract
Cerebral ischemia-reperfusion (I/R) can result in severe brain injury, for which there are no optimal treatment options. I/R is often accompanied by increased autophagy. Beclin-1, a central player in autophagy, has been extensively studied in I/R; however, to date, at least to the best of our knowledge, there are no definitive descriptions of its specific role. Thus, the aim of the present study was to explore the regulatory role played by Beclin-1 in I/R. In vivo experiments were performed using an animal model of brain I/R with male Sprague-Dawley rats. Brain tissue damage was observed using 2,3,5-triphenyltetrazolium chloride, and hematoxylin and eosin staining. Tissue apoptosis levels were evaluated using a TUNEL assay, as well as western blot analysis. Immunofluorescence together with western blot analysis was used to detect autophagy in the tissues. Immunohistochemistry and western blot analysis were used to analyze DNA double-stranded breaks (DSBs). Moreover, HT22 cells overexpressing Beclin-1 were subjected to oxygen glucose deprivation/reoxygenation injury to simulate I/R pathological damage in vitro. Apoptosis was assessed using TUNEL and flow cytometric assays in this in vitro model, and autophagy was assessed using immunofluorescence and western blot analysis. The DSBs of the cells were analyzed using western blot analysis. I/R activated autophagy and induced DSBs. Autophagy inhibitors decreased brain tissue damage and reduced cell apoptosis; however, the degree of decrease in damage and apoptosis was not highly associated with the change in autophagy, and the frequency of DSBs slightly increased. The overexpression of Beclin-1 in neurons significantly attenuated I/R-induced damage and promoted DSB repair. On the whole, the present study demonstrates that Beclin-1 protects neurons from ischemic damage through the non-autophagy-dependent regulation of DNA repair processes.
Keywords: Beclin-1, ischemia/reperfusion, autophagy, DNA repair function, neuroprotection
Introduction
Cerebral ischemia usually occurs as a result of a disruption of the blood supply, leading to a decrease in the supply of glucose and oxygen to brain tissues. Following the restoration of the supply, the return of blood flow can result in secondary brain tissue damage (1). The progression of cerebral ischemia/reperfusion (I/R) is dependent on the interaction of several mechanisms, such as the production of free radicals, glutamate over-release and calcium overload (2,3). Early during this process, due to the elevated oxygen supply, the lesioned area rapidly produces large quantities of oxygen radicals, and these toxic products are the main cause of DNA damage (4,5). DNA double-stranded breaks (DSBs) are the primary form of DNA damage and are one of the hallmarks of cell death (6,7). It has been previously demonstrated that DNA repair is increased in ischemic neurons, producing an endogenous repair effect (8,9). The enhancement of the effective repair of damaged DNA and the inhibition of cell death may therefore have a positive protective effect against central nervous system-damaging diseases, such as ischemic stroke.
Autophagy is a procedure that selectively degrades cellular components to maintain the balance of the internal environment (10). Being an essential molecule in autophagosome formation, Beclin-1 mediates the localization of other autophagic proteins to phagocytic vesicles, thereby regulating the formation and maturation of mammalian autophagosomes (11,12). Beclin-1 expression is typically elevated and activates autophagy in stroke and cerebral I/R injury. It has been shown that homocysteine accumulation in a brain model of I/R can promote the release of Beclin-1 and the apoptosis of neuronal cells, enhance DNA damage and induce neuronal death; this indicates that, on the one hand, Beclin-1, apoptosis and DNA damage are positively associated, and on the other hand, the high expression level of Beclin-1 is positively linked to severe I/R injury (13). On the contrary, a protective role of Beclin-1 in I/R injury was suggested by another study which demonstrated that increased levels of Beclin-1 activated autophagy and contributed to neuroprotection against focal cerebral ischemia in rats (14). However, from the current evidence, no definite conclusions have yet been reached as regards the role of Beclin-1 in I/R, and autophagy and apoptosis may not be sufficient to fully explain the contradictory role of Beclin-1 in I/R injury. It is worth noting that there is a close association between Beclin-1 and DNA damage repair. It has been demonstrated that the knockdown of Beclin-1 significantly blocks the formation of DNA-dependent protein kinase (DNA-PK) complexes, while the Mre-11 (DSB repair protein), Nijmegen breakage syndrome 1 and Rad-50 (DNA repair protein) (MNR) complex is slightly affected, both of which are crucial for repairing damage to DSBs. This suggests that Beclin-1 can influence DNA repair ability by regulating the expression of several DSB repair proteins and the formation of repair complexes (15,16).
At present, there remain unresolved issues and controversies as regards the physiological and pathological roles of the increase and decrease in Beclin-1 expression levels following cerebral I/R injury. Considering the close connection between Beclin-1 and DNA damage, as well as the close association among DNA damage, apoptosis and I/R injury as aforementioned, the present study thus aimed to further investigate the role and regulatory mechanisms of Beclin-1 in DNA damage, autophagy and apoptosis following cerebral I/R injury.
Materials and methods
Animal model of cerebral I/R
A total of 27 male Sprague-Dawley (SD) rats weighing 220-260 g and aged 7-8 weeks (Guangxi University Animal Experiment Center, Guangxi, China) were used. All experiments were approved by the Ethics Committee of the Affiliated Hospital of Youjiang Medical University for Nationalities (approval no. YYFY-LL-2020-17), and performed in accordance with the guidelines detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health) (17). All animals were housed at 22±2°C, with 50-60% humidity, a 12-h light/dark cycle and were provided with ad libitum access to food and water.
The rat model of cerebral I/R was established as previously described (18). The rats were anesthetized by an intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight; Sigma-Aldrich; Merck KGaA) after 12 h of fasting. Following that, the dermatome was incised slightly to the right along the middle of the neck, and the right common carotid artery (CCA), right internal carotid artery (ICA) and right external carotid artery (ECA) were bluntly dissected. Surgical wires (5-0) were ligated proximal to the CCA and proximal to the ECA. Upon clamping the ICA, a small incision was made at the distal end of the ECA, and a wire plug (the diameter of the wire plug was selected according to the weight of the rat) was inserted towards the ICA at a depth of 16-20 mm, causing a flow block in the middle cerebral artery and ligating the ICA. The removal of the wire bolus caused reperfusion 90 min later. The sham-operated (sham) group underwent the same surgery, but without cerebral artery occlusion. The anal temperature of the rats was maintained at 37±0.5°C throughout the procedure with an infrared heat lamp and a heating pad. All rats were randomly divided into three groups (n=9 per group): The sham group, model group and 3-methyladenine (3-MA) group. Rats in the 3-MA group were injected with 10 µl 3-MA at 600 nmol, dissolved in normal saline (MedChemExpress), a classical autophagy inhibitor, to the lateral ventricles with a microinjector 60 min prior to modeling. Moreover, the rats in the sham and model groups received an equivalent volume of normal saline. After 24 h of modeling, the rats were euthanized by an intraperitoneal injection of pentobarbital sodium (200 mg/kg body weight) prior to collecting the brain tissues. Afterwards, 3 rats were used for 2,3,5-triphenyltetrazolium chloride (TTC) staining. In the remaining 6 rats, the right brain tissues were collected for use in western blot analysis, and the left brain tissues were collected for analysis using staining: In total, three left brain tissues were used for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC), and the remaining three left brain tissues were collected for immunofluorescence (IF) and TUNEL assay.
In vitro model of oxygen-glucose deprivation/reperfusion (OGD/R)
Mouse hippocampal cells (HT22; MilliporeSigma) were used in all the in vitro experiments. The cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.), enclosed in a 5% CO2-95% O2, 37°C constant temperature incubator.
The OGD/R model was used to simulate I/R injury in vitro. Briefly, the cell culture medium was replaced with glucose-free Earle's solution (Gibco; Thermo Fisher Scientific, Inc.) and the cells were incubated in a hypoxic incubator (1% O2, 5% CO2, 94% N2) at 37°C for 4 h. Subsequently, the medium was changed to normal medium and the cells were incubated under normoxic atmospheric conditions (5% CO2; 95% air) with reoxygenation for 12, 24 or 48 h. The cells were divided into five groups as follows: The control, model, 3-MA, 3-MA + overexpression-negative control (OE-NC) and 3-MA + Beclin-1 overexpression (OE-BECN1) groups. The cells in all groups, apart from those in the control and model groups were treated with 3-MA (10 mM), which was added to the culture medium prior to reoxygenation.
Cell transfection
Transfection was performed when the cells were 80% confluent prior to the OGD/R experiments. OE-BECN1#1/2 and 0.8 µg OE-NC (Shanghai GenePharma, Co., Ltd.) vectors at a final concentration of 50 nM were transfected into the cells using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h at 37°C according to the manufacturer's instructions. At 48 h post-transfection, the cells were used for subsequent experiments.
Reverse transcription-quantitative PCR (RT-qPCR) was used to assess the mRNA expression levels of BECN1 in the cells in order to verify successful transfection. RNA extraction and subsequent reverse transcription were performed according to the manufacturer's instructions and as described below.
RT-qPCR
The extraction of RNA from cells was performed using TRIzol® reagent (Thermo Fisher Scientific, Inc.). The reverse transcription of individual RNA samples was then performed using total RNA and a RevertAid First Strand DNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol as follows: 25°C for 5 min, 42°C for 60 min and 70°C for 5 min. The newly synthesized first-strand cDNA was ready for immediate downstream applications, or for long-term storage at -80°C. mRNA levels in all samples were measured using qPCR with the DreamTaq Green PCR MasterMix kit (Thermo Fisher Scientific, Inc.) on a CFX Connect fluorescent quantitative PCR instrument (Bio-Rad Laboratories, Inc.). The RT-qPCR conditions were as follows: Pre-denaturation at 95°C for 2 min; followed by 35 cycles of 95°C for 35 sec, 58°C for 45 sec and 72°C for 30 sec; and 72°C for 5 min. Relative expression levels were calculated using the 2−ΔΔCq method with GAPDH as the housekeeping gene (19). The primer sequences used were as follows: Beclin-1 forward, 5′-GCT GTA GCC AGC CTC TGA AA-3′ and reverse, 5′-AAT GGC TCC TGT GAG TTC CTG-3′; and GAPDH forward, 5′-AAG AAG GTG GTG AAG CAG G-3′ and reverse, 5′-GAA GGT GGA AGA GTG GGA GT-3′.
TCC staining
Following sacrifice, the rat brains were removed, and six consecutive 2-mm-thick coronal sections were prepared to examine the infarct volume in the model rats. The sections were completely immersed in 2% TTC staining solution (Jiancheng Bioengineering Institute) for 30 min at room temperature, protected from light, and then fixed in 4% paraformaldehyde at room temperature; the infarcted tissue appeared white. Images were acquired using a digital camera (Canon, Inc.) and analyzed using ImageJ version 4.6.2 (National Institutes of Health). The infarct volume was calculated by multiplying the added infarct areas of each slices by slice thickness, and the results were presented as a ratio of (infarct volume/the whole brain volume) ×100%
H&E staining
At 24 h after modeling, the rats were euthanized by an intraperitoneal injection of pentobarbital sodium (200 mg/kg body weight) prior to harvesting the brain tissue. After fixing in 4% paraformaldehyde, the brain tissues were paraffin-embedded and cut into sections (4-µm-thick). The H&E staining kit (Beyotime Institute of Biotechnology) was then used and the experimental procedure strictly adhered to the manufacturer's instructions as follows: The sections were stained with hematoxylin solution at 37°C for 5 min and stained with eosin solution at 37°C for 3 min. The sections were finally observed under a light microscope (Nikon Corporation); five fields of view were randomly selected for analysis in each group.
TUNEL assay
All operations were performed according to the instructions provided with the TUNEL Apoptosis Assay kit (Beyotime Institute of Biotechnology). After returning the frozen brain tissue sections to room temperature, they were fixed in 4% paraformaldehyde for 30 min at room temperature, and permeabilized with PBS containing 0.5% Triton X-100 for 5 min. The tissues were then incubated with TUNEL reaction mixture for 60 min at 37°C, protected from light. After blocking with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Beijing Solarbio Science & Technology Co., Ltd.), five random fields of view were observed under a fluorescence microscope (Nikon Corporation; magnification, ×200).
Western blot analysis
Brain tissues or HT22 cells were homogenized using RIPA lysis reagent (Beyotime Institute of Biotechnology) containing protease inhibitors and phosphatase inhibitors to extract total protein. The protein content was determined using a Bradford assay (MilliporeSigma), according to the manufacturer's instructions. Proteins (20 µg/lane) were immobilized onto PVDF (MilliporeSigma) membranes using 8-12% SDS-PAGE (Beyotime Institute of Biotechnology). The PVDF membranes were blocked with 5% BSA at room temperature for 1 h and incubated with the corresponding primary antibodies overnight at 4°C. Subsequently, the membranes were incubated with the secondary antibody (1:2,000; cat. no. ab96899, Abcam) at room temperature for 2 h. Signals were visualized using ECL reagent (Thermo Fisher Scientific, Inc.) and the signal intensity was measured using ImageJ software (version 1.48v; National Institutes of Health). The primary antibodies used were the following: Bax (1:1,000; cat. no. ab32503, Abcam), Bcl-2 (1:1,000; cat. no. ab182858, Abcam), cleaved caspase-3 (1:1,000; cat. no. 9661, Cell Signaling Technology, Inc.), Beclin-1 (1:500; cat. no. ab62557, Abcam), autophagy related (ATG)5 (1:1,000; cat. no. ab221604, Abcam), ATG7 (1:1,000; cat. no. ab133528, Abcam), light chain 3 (LC3; 1:500; cat. no. 43566, Cell Signaling Technology, Inc.), γ histone family member X (γH2AX; 1:500; cat. no. ab81299, Abcam), DNA-PK (1:1,000; cat. no. ab32566, Abcam) and GAPDH (1:1,000; cat. no. ab181602, Abcam).
IF assay
Cells or brain tissue sections were fixed with 4% paraformaldehyde at room temperature and permeabilized with 1% Triton X-100 at room temperature and then blocked with 5% BSA for 1 h. Subsequently, cells/tissues were incubated overnight at 4°C with the corresponding primary antibodies (NeuN; 1:200; cat. no. 94403 or LC3; 1:200; cat. no. 43566; Cell Signaling Technology, Inc.). The following day, goat anti-rabbit IgG H&L/HRP (1:200; BIOSS; cat. no. bs-40295G-HRP) and rabbit anti-mouse IgG-Fc/FITC antibodies (1:200; BIOSS; cat. no. bs-0377R-FITC) were co-incubated with the cells/tissues at room temperature for 1 h. The nuclei were stained with DAPI (Jiancheng Bioengineering Institute) for 15 min at room temperature. The samples were washed with PBS between all steps. The results were observed under a fluorescence microscope (Olympus BX51; Olympus Corporation) with five random fields of view per group.
IHC
An IHC detection system kit (BIOSS) was used in the present study. The brains sections were deparaffinized and rehydrated, followed by antigen retrieval and cooling to room temperature. The sections were then immersed in 3% H2O2-methanol for 10 min, blocked dropwise with 10% goat serum, and incubated with the primary antibody (γH2AX, 1:500; cat. no. ab124781; Abcam) at 4°C overnight. According to the manufacturer's instructions, anti-rabbit lgG HRP-conjugated secondary (1:2,000; cat. no. ab205718; Abcam) for 1 h at room temperature were added and 3,3′-diaminobenzidine tetrahydrochloride (DAB; Beijing Solarbio Science & Technology Co., Ltd.) was used for chromogenic development.
Cell Counting Kit-8 (CCK-8) assay
The activity of the OGD/R cells following treatment with 3-MA and transfection to induce Beclin-1 overexpression was investigated using a CCK-8 assay (Beyotime Institute of Biotechnology) for 2 h of culture at 37°C, according to the manufacturer's protocol, and the absorbance at 450 nm was measured used using a microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometric analysis for apoptosis
Apoptosis was detected using an Annexin V-FITC Apoptosis Detection kit (eBioscience; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The cells were resuspended in binding buffer at a density of 5×105 cells/ml, mixed with 5 µl Annexin V-FITC per 195 µl binding buffer, incubated for 10 min at room temperature, washed with binding buffer and then resuspended with 190 µl binding buffer, adding 10 µl propidium iodide (20 µg/ml). The apoptosis of the cells was analyzed using a flow cytometer (FC500; Beckman Coulter, Inc.).
Statistical analysis
All data were statistically analyzed using SPSS software version 18.0 (SPSS, Inc.), and data are presented as the mean ± standard deviation. Differences between multiple groups were analyzed using one-way ANOVA, followed by Tukey's post hoc test. GraphPad Prism version 8.0 (GraphPad Software, Inc.) was used for plotting data. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of autophagy partially attenuates I/R-induced brain tissue lesions
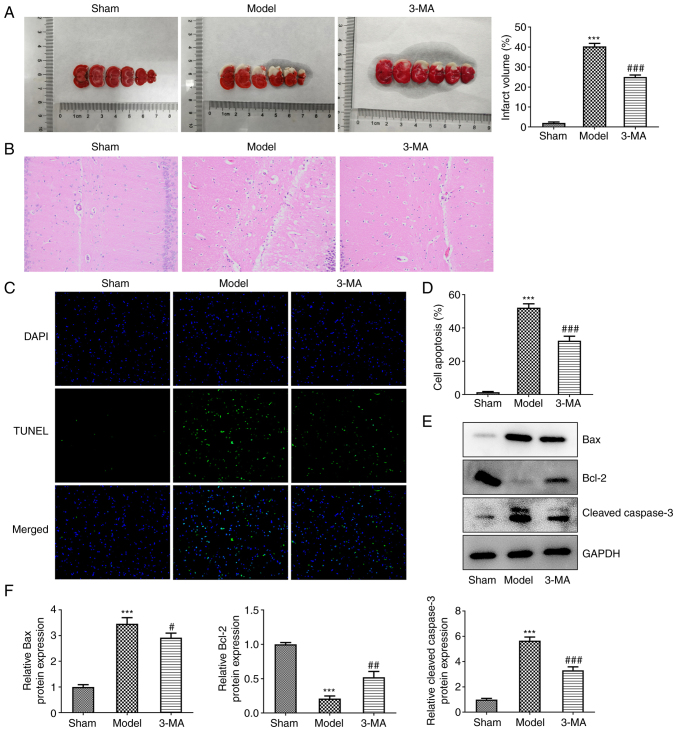
A rat model of focal cerebral I/R was established and the autophagy inhibitor, 3-MA, was administered for experimental validation. In comparison with the model group, the results of TTC staining revealed that the area of cerebral infarction (brain areas remaining white) was notably reduced in the 3-MA group (25.00±1.00 vs. 40.33±1.53, P<0.001) (Fig. 1A). As also revealed using H&E staining, the cells in the sham group were uniformly colored, densely distributed, neatly arranged, with a full cytosol, intact cell structures and visible nucleoli (Fig. 1B). However, the cells in the model group were disordered, with a reduced cytosolic volume, densely stained cytoplasm, loose tissue and unclear nuclear membranes. The 3-MA group exhibited an altered cellular state with more ordered cell alignment, fewer tissue voids and fewer nuclear alterations. The pathological results thus revealed that the inhibition of autophagy significantly reduced cerebral I/R damage to brain tissue, enhancing neuroprotection.
Figure 1.
Inhibition of autophagy attenuates ischemia-reperfusion injury in rat brains. (A) 2,3,5-Triphenyltetrazolium chloride staining and infarct volume; n=3. (B) Hematoxylin and eosin staining; n=3. (C and D) TUNEL assay was used to detect the level of apoptosis; n=3. (E and F) Western blot analysis of apoptosis-related proteins; n=6. ***P<0.001 vs. sham group; #P<0.05, ##P<0.01 and ###P<0.001 vs. model group. Sham, sham-operated group; 3-MA, 3-methyladenine.
Apoptosis was analyzed using a TUNEL assay, as well as western blot analysis. In the TUNEL assay, apoptotic cells exhibited a fluorescent green color (Fig. 1C and D). The inhibition of autophagy decreased the apoptosis of brain neuronal cells, compared with the model group (32.33±2.72 vs. 52.17±2.40, P<0.001). This was validated by the subsequent quantitative analysis of apoptosis-related proteins. The results of western blot analysis revealed that cerebral I/R injury led to an increase in the expression of the the pro-apoptotic proteins, Bax (3.46±0.24 vs. 1.00±0.09, P<0.001) and cleaved-caspase-3 (5.66±0.28 vs. 1.00±0.09, P<0.001), with a decrease in the expression of the anti-apoptotic protein, Bcl-2 (0.21±0.04 vs. 1.00±0.03, P<0.001) (Fig. 1E and F). By contrast, pre-treatment with 3-MA was able to partially reverse the disorganized expression levels of both classes of apoptosis-related proteins (2.92±0.18 vs. 3.46±0.24, P<0.05 for Bax; 3.32±0.27 vs. 5.66±0.28, P<0.01 for cleaved caspase-3; and 0.52±0.08 vs. 0.21±0.04, P<0.001 for Bcl-2).
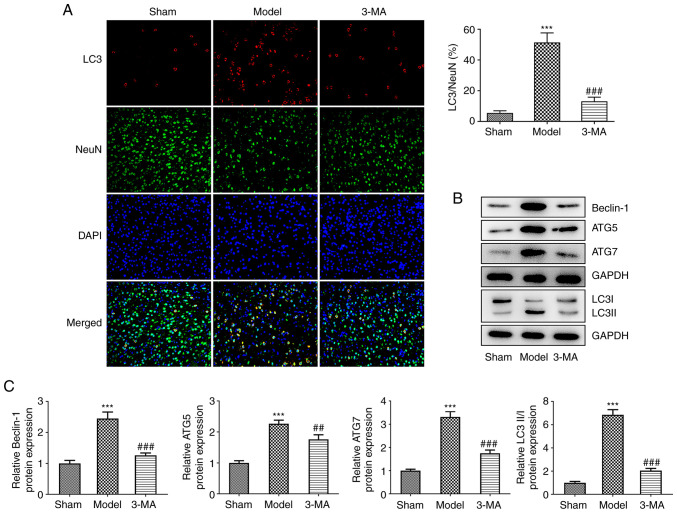
The brain sections following I/R injury were specifically stained for the key autophagy protein, LC3, as well as the neuronal marker, NeuN, which revealed that I/R injury resulted in an increase in LC3 and NeuN fluorescent double-positive cells, and the ratio of LC3 to NeuN-positive cells was markedly upregulated compared to the sham group, indicating that LC3 was primarily expressed in the cytoplasm of neurons, and autophagy induced by I/R occurred primarily in neurons (Fig. 2A). Following pre-treatment with 3-MA, a decrease in neuronal autophagy levels was observed, characterized by a significant decrease in LC3 and NeuN fluorescence double-positive staining. In addition, the results of IF assay demonstrated that 3-MA upregulated the number of NeuN-positive cells, suggesting that the inhibition of autophagy reduced neuronal death induced by I/R. Additionally, using western blot analysis of autophagy-related proteins (Fig. 2B and C), it was found that the levels of Beclin-1 (2.45±0.21 vs. 1.00±0.10, P<0.001), ATG5 (2.27±0.11 vs. 1.00±0.07, P<0.001), ATG7 (3.31±0.24 vs. 1.00±0.06, P<0.001) and LC3II/LC3I (6.86±0.44 vs. 1.00±0.11, P<0.001) were increased in the brain tissue of rats following I/R injury compared with the sham group; I/R injury also significantly promoted the development of autophagy in the brain. This process was greatly antagonized by 3-MA. Taken together, these results suggested that I/R significantly enhanced autophagy in the brain and contributed to neuronal cell death, whereas 3-MA administration prior to modeling inhibited autophagy and thus attenuated the damage induced by cerebral ischemia-reperfusion. Such protective effects were however, limited and the damage observed was still greater than that in the control group.
Figure 2.
3-MA significantly reduces brain ischemia-reperfusion-induced autophagy. (A) Immunofluorescence staining for LC3 (red), NeuN (green) and DAPI (blue) in the hippocampal areas of each group; n=3. (B and C) Western blot analysis of cellular autophagy-associated proteins; n=6. ***P<0.001 vs. sham group; ##P<0.01 and ###P<0.001 vs. model group. Sham, sham-operated group; 3-MA, methyladenine; LC3, light chain 3.
Inhibition of autophagy may exacerbate DNA damage induced by cerebral I/R
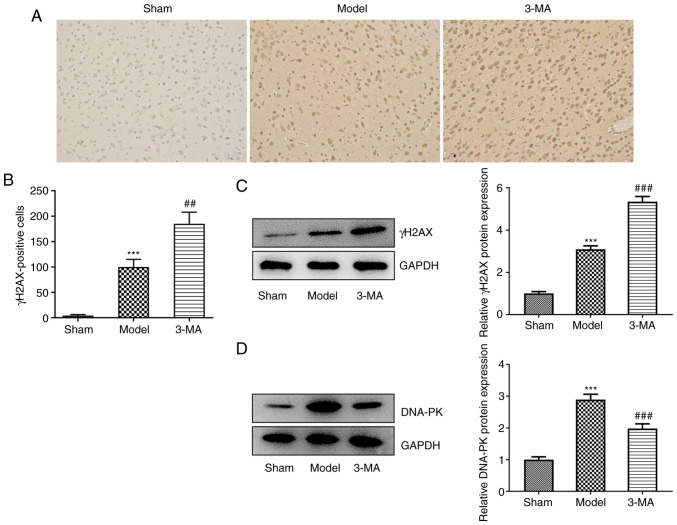
γH2AX is considered a biological marker for the detection of DSBs (20). In the present study. IHC staining of the rat brain sections was performed to assess the protein expression of γH2AX in each group as a reflection of cellular DSBs (Fig. 3A and B). It was found that the number of cells specifically immunostained for γH2AX was significantly increased compared with the sham group, suggesting that I/R induced a substantial increase in the degree of cellular DSBs, which may have been responsible for neuronal cell death. However, the addition of 3-MA, instead of ameliorating the severe cellular DNA damage observed, further increased the degree of DSBs. Furthermore, western blot analysis clearly demonstrated an increase in the protein expression levels of γH2AX in the 3-MA group compared with the model group (5.35±0.25 vs. 3.10±0.16, P<0.001) (Fig. 3C). This may suggest that the inhibition of autophagy, whilst possibly reducing brain tissue damage to a certain extent, also impeded DNA damage repair.
Figure 3.
Inhibition of autophagy may exacerbate DNA damage induced by cerebral ischemia-reperfusion. (A and B) Immunohistochemistry assay; n=3. (C and D) Western blot analysis of γH2AX and DNA-PK expression; n=6. ***P<0.001 vs. sham group; ##P<0.01 and ###P<0.001 vs. model group. Sham, sham-operated group; 3-MA, methyladenine; DNA-PK, DNA-dependent protein kinase; γH2AX, γ histone family member X.
The results of the analysis of DNA-PK expression also supported this view. DNA-PK belongs to the phosphatidylinositol 3-kinase-related kinase family, and is most well-known for its role in the repair of DSBs (20). The quantitative protein analysis of DNA-PK in each group revealed that brain injury induced an endogenous repair mechanism, as indicated by a significant increase in DNA-PK protein expression in the model group compared with the sham group (2.90±0.17 vs. 1.00±0.09, P<0.001); however, this endogenous repair mechanism was diminished by pre-treatment with 3-MA (1.98±0.14 vs. 2.90±0.17, P<0.001) (Fig. 3D).
Combining brain tissue damage and DNA damage studies, it was found that while the inhibition of autophagy could lead to a certain degree of reduction in tissue damage, DNA damage was not reduced, but instead increased significantly. Thus, although Beclin-1 plays a central role in autophagy, it may also affect the repair of DSBs by influencing the formation of DNA-PK. Combined with this background, it was hypothesized that Beclin-1 itself had mechanisms related to the induction of repair of DSBs resulting in the aforementioned experimental results, and that although 3-MA inhibited autophagy, it also inhibited the expression of Beclin-1, and thus its induction of DSB repair; thus, the tissue damage remained at a higher level, not decreasing as markedly as the autophagy level itself.
Overexpression of Beclin-1 attenuates OGD/R-induced cell damage independently of autophagy, and this is further augmented by 3-MA treatment
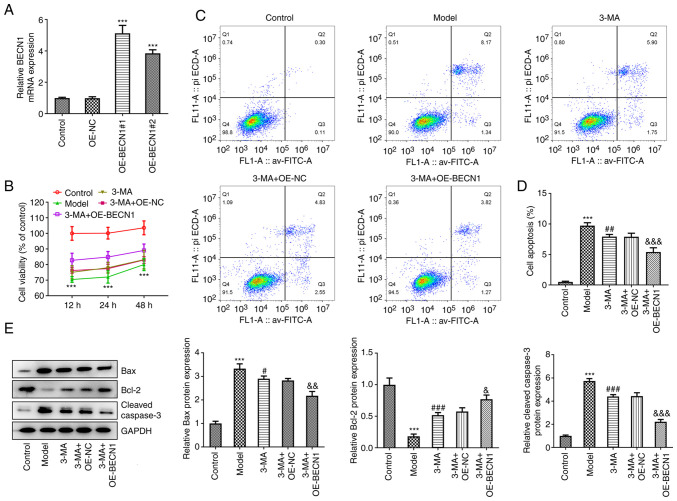
In vitro cell experiments were performed to determine the effects of Beclin-1 overexpression on OGD/R-induced cell damage. Initially, a Beclin-1 overexpression vector was constructed and transfected into HT22 cells. RT-qPCR revealed that the overexpression was successful (5.12±0.51 and 3.84±0.23 vs. 0.99±0.09, P<0.001) (Fig. 4A). The more efficient OE-BECN1#1 was selected for use in subsequent experiments. An OGD/R cell model was prepared for subsequent experiments. The results of CCK-8 assay revealed that, consistent with the results of the in vivo experiments, pre-treatment with 3-MA enhanced the activity of cells exposed to OGD/R injury to a modest level; transfection with the blank plasmid did not alter the effects of 3-MA (Fig. 4B). The 3-MA + OE-BECN1 group exhibited a pronounced elevation in cellular activity compared with the 3-MA + OE-NC group. Moreover, the cells in the model group were in the lowest activity state following 12 h of reoxygenation; thus the time duration of 12 h of reoxygenation was selected for use in subsequent experiments.
Figure 4.
High expression of Beclin-1 significantly attenuates oxygen-glucose deprivation/reperfusion-induced neuronal injury. (A) Reverse transcription-quantitative PCR was performed to evaluate the plasmid transfection efficiency. (B) CCK-8 assay. (C and D) Flow cytometry was performed to evaluate apoptosis. (E) Western blot analysis of cellular autophagy-associated proteins. ***P<0.001 vs. control; #P<0.05, ##P<0.01 and ###P<0.001 vs. model group; &P<0.05, &&P<0.01 and &&&P<0.001 vs. 3-MA + OE-NC group. 3-MA, methyladenine; OE-NC, overexpression-negative control; OE-BECN1, Beclin-1 overexpression vector.
The degree of apoptosis in each group was subsequently analyzed. As demonstrated by the results of flow cytometric analysis, 3-MA inhibited autophagy and was able to reduce apoptosis following injury (7.93±0.36 vs. 9.74±0.47, P<0.01). In the cells overexpressing Beclin-1 and treated with 3-MA, the degree of inhibition of apoptosis was more potent than that observed in the cells treated with 3-MA and transfected with the blank vector (5.40±0.70 vs. 7.90±0.58, P<0.001) (Fig. 4C and D). In the quantitative analysis of proteins in each group, the 3-MA + OE-BECN1 group exhibited a significant decrease in the expression levels of Bax (2.17±0.19 vs. 2.83±0.08, P<0.01) and cleaved caspase-3 (2.23±0.19 vs. 4.44±0.29, P<0.001), and a significant increase in the levels of Bcl-2 compared with the 3-MA + OE-NC group (0.77±0.07±0.58±0.06, P<0.05) (Fig. 4E).
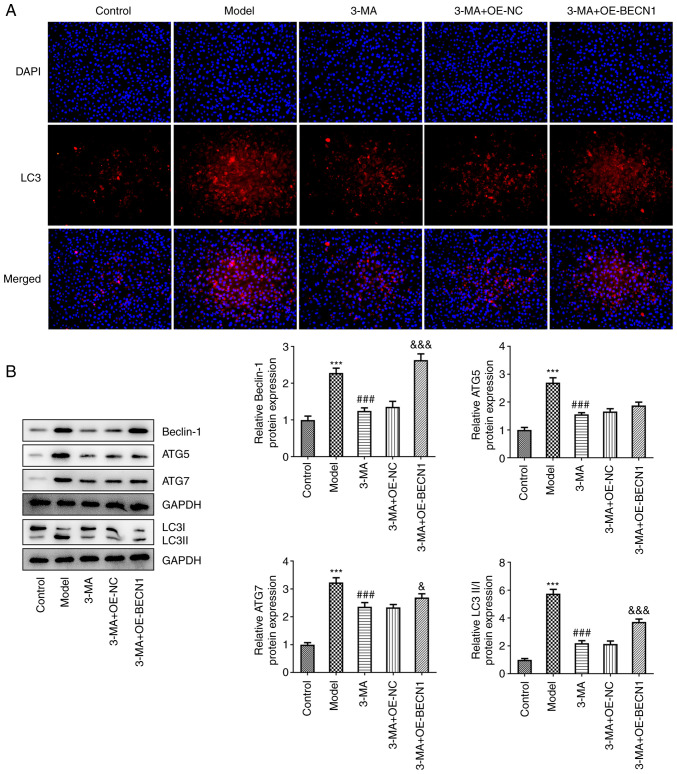
The levels of autophagy in each group were also measured. The results of IF assay revealed that there was a notable enhancement in the fluorescence intensity of LC3 in the 3-MA + OE-BECN1 group (Fig. 5A). The results of western blot analysis also revealed that Beclin-1 expression in the 3-MA + OE-BECN1 group was significantly increased compared with the group transfected with the blank vector (2.63±0.17 vs. 1.36±0.15, P<0.001); in addition, the expression of ATG7 (2.69±0.13 vs. 2.34±0.11, P<0.05) and the LC3II/LC3I ratio (3.72±0.20 vs. 2.14±0.21, P<0.001) in the 3-MA + OE-BECN1 group also moderately increased; the levels of ATG5 were also increased, alhtough this increase was not significant (1.88±0.12 vs. 1.66±0.10, P=0.225) (Fig. 5B). In the 3-MA group, the overexpression of BECN1 limited the expression of autophagy-related proteins.
Figure 5.
High expression of Beclin-1 leads to a rebound in autophagy in response to 3-MA inhibition to a certain extent. (A) Immunofluorescence staining for LC3 (red) and DAPI (blue) in the hippocampal areas of each group. (B) Western blot analysis of cellular autophagy-associated proteins. ***P<0.001 vs. control; ###P<0.001 vs. model group; &P<0.05 and &&&P<0.001 vs. 3-MA + OE-NC group. 3-MA, methyladenine; OE-NC, overexpression-negative control; OE-BECN1, Beclin-1 overexpression vector.
Thus, it could be concluded that despite the modest increase in autophagy, the overexpression of Beclin-1 exerted a pronounced cytoprotective effect, suggesting that this effect may not be dependent on the cellular autophagic pathway.
Beclin-1 augments DNA repair independently of autophagy
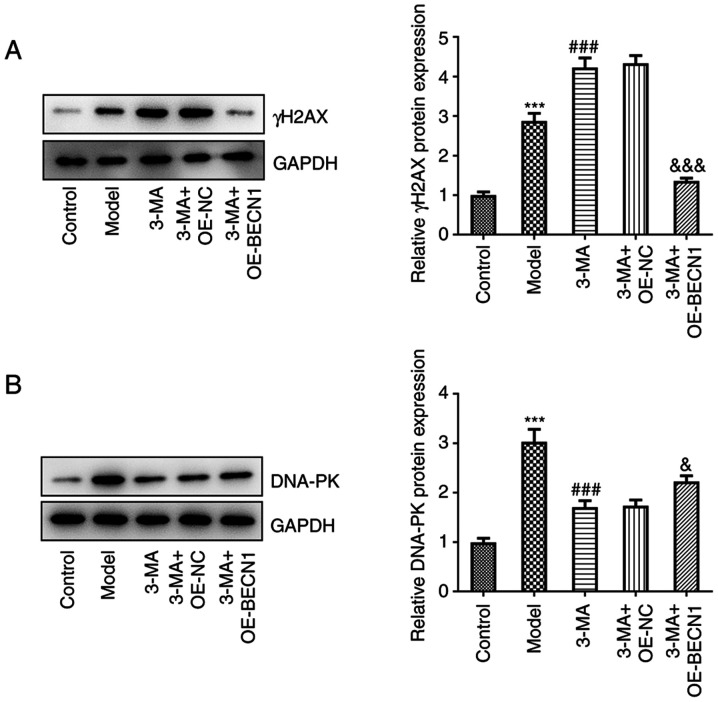
To investigate whether Beclin-1 itself can affect DNA DSB repair, and whether this effect is dependent on the regulation of cellular autophagy, the γH2AX and DNA-PK levels were quantified in each group of cells (Fig. 6A and B). In the cells treated with 3-MA, the high expression levels of Beclin-1 potently suppressed the levels of γH2AX compared with the group transfected with the blank vector (1.35±0.08 vs. 4.33±0.20, P<0.001), and there was a rebound in the weakened DNA-PK formation (2.22±0.12 vs. 1.74±0.12, P<0.05). This indicated that the high expression of Beclin-1, although causing a minimal decrease in intracellular autophagy, exerted a potent repairing effect on DSBs under 3-MA treatment; this repairing effect which occurred asynchronously with the autophagy levels may be attributed to the fact that Beclin-1 itself plays a role in repairing DSBs that may be independent of the autophagic pathway.
Figure 6.
Beclin-1 is able to promote DNA repair independently of autophagy. (A and B) Western blot analysis of γH2AX and DNA-PK expression. ***P<0.001 vs. control; ###P<0.001 vs. model group; &P<0.05 and &&&P<0.001 vs. 3-MA + OE-NC group. DNA-PK, DNA-dependent protein kinase; γH2AX, γ histone family member X; 3-MA, methyladenine; OE-NC, overexpression-negative control; OE-BECN1, Beclin-1 overexpression vector.
Discussion
In the present study, a rat brain I/R model and OGD/R neuronal model were established to simulate cerebral I/R injury in vitro and in vivo. The findings revealed that cerebral I/R significantly induced high levels of autophagy and neuronal injury, whereas the autophagy inhibitor, 3-MA, was able to partially prevent brain injury, but also aggravated DSBs. The overexpression of Beclin-1 resulted in an increase in the expression levels of anti-apoptotic proteins, whilst promoting the synthesis of DNA repair proteins, and these roles were not associated with its ability to regulate autophagy. It is clearly evident from these results that Beclin-1 protects neurological function in the brain following I/R injury by improving the DNA damage repair capacity of cells, independent of the regulation of autophagy.
The accumulation of sudden bursts of oxides in I/R can cause DNA breaks and lead to the necrosis of ischemic neurons (6,21,22). In fact, it has been found in previous studies that the levels of DNA repair increase following transient ischemia (6,9). In the present study, it was likewise confirmed that the γH2AX levels, which are characteristic of DSBs, were significantly elevated in response to stimuli; the increased synthesis of DNA-PK also demonstrated that the organism itself initiated a repair mechanism. In this view, the effective improvement of the organism's DNA repair capacity enhanced neuroprotection. It appears that the effective enhancement of endogenous DNA repair enhances neuroprotection.
Autophagy is the process through which cells are targeted by lysosomes to recycle functionally damaged organelles, as well as misfolded proteins (23). Moderate autophagy facilitates endogenous repair, and it has been shown that I/R can ameliorate neuronal damage through mitochondrial clearance via the activation of autophagy (24). However, the excessive activation of autophagy may lead to cell death. For example, it has been reported that the inhibition of autophagy can shrink the infarct lesion volume and exerts a neuroprotective effect in rats with cerebral I/R injury (25). Additionally, sevoflurane has been reported to protect the brain against injury by inhibiting autophagy in rats with cerebral I/R (26). On the contrary, eugenol has been shown to attenuate cerebral I/R injury by enhancing autophagy (27). Herein, it was found that the lesioned areas exhibited a high level of autophagy in affected neurons, accompanied by more severe tissue and cellular damages, which may be responsible for neurodegeneration; 3-MA inhibited autophagy, reduced the infarct volume and neuronal apoptosis, and protected the nerve cells. However, although the addition of 3-MA reduced brain tissue and neuronal damage, the amplitude of the reduction did not correspond with the degree of the decrease in autophagy, and the amount of damage remained relatively high. In addition, it was also observed that 3-MA resulted in more severe DSBs than the model group, as evidenced by an increase in γH2AX levels and a reduction in DNA-PK levels, which may explain why the inhibition of autophagy alone did not improve neurological function following I/R in the brain.
Beclin-1 is an essential autophagy regulatory gene. Although studies on the functional regulation of Beclin-1 through autophagy have dominated prior efforts, increased attention is currently being paid to its role in non-autophagy-dependent pathways (15). It has been shown that the non-autophagy-dependent effects of Beclin-1 manifest in the antagonism of growth factors (28,29), the repair of cellular DNA (15) and the aggregation of cellular chromosomes (30). In this regard, of great interest is the association between Beclin-1 and DNA damage repair. In the study by Xu et al (15), it was found that cells undergo severe DNA damage during phase-responsive radiation, Beclin-1 nuclear localization is augmented and intra-nuclear Beclin-1 is able to interact with DNA topoisomerase IIβ to recruit it toward DNA break sites; the overexpression of Beclin-1 in wild-type cells with physiologically normal autophagy enhances autophagy and improves DNA repair, and in the absence of both, Beclin-1 will not function. Of interest, in the present study, it was demonstrated that 3-MA reduced the autophagy levels, and inhibited Beclin-1 expression in brain tissue, as well as in cells, affecting its regulation of DNA repair, thus causing more severe DNA damage than in the model group. The overexpression of Beclin-1 in cells with 3-MA-restricted autophagy and exposure to ODG/R resulted in a decrease in Bax and in cleaved caspase-3 expression levels. Bax enhanced the caspase-mediated cleavage of Beclin-1, thereby inducing apoptosis. Additionally, the overexpression of Beclin-1 induces a significant increase in endogenous Bcl-2 content, as demonstrated by several studies assessing the protective effects of Bcl-2 on neurons from ischemic challenge and the enhanced neuroprotection imbued (24,31,32). In the present study, the level of autophagy was slightly recovered by the overexpression of Beclin-1, and a significant decrease in γH2AX levels, as well as a notable increase in the DNA-PK complex were observed during the same period. This indicated that the overexpression of Beclin-1 could correct the repair defects of DSBs under 3-MA pre-treatment, and the degree of increase in repair was not associated with the degree of recovery of autophagy. This highlights the potential involvement of Beclin-1 in regulating DNA repair independently, rather than via autophagy.
Overall, to the best of our knowledge, the present study is the first to examine the non-autophagic role of Beciln-1 in cerebral I/R, and Beclin-1 was shown to enhance neuronal DNA repair to resist I/R damage through a non-autophagy-dependent regulatory mechanism. Additionally, the present study demonstrated that the inhibition of the excessive activation of autophagy combined with Beclin-1 upregulation was more effective in mitigating I/R injury than autophagy inhibition alone. The findings of the present study may contribute to the investigation of the mechanisms of I/R injury, and may assist in improving the therapeutic effects, providing a novel perspective for studying the role of Beclin-1 and autophagy. Nevertheless, the present study was preliminary in its nature, and did not validate the effects of the overexpression of Beclin-1 on DNA repair under the complete inhibition of autophagy, which will thus form the basis of future studies. In addition, the use of primary neurons as experimental subjects and in vivo verification can further validate the results, which needs to be taken into consideration in future studies.
Acknowledgments
Not applicable.
Funding Statement
The present study was supported by the National Key R&D Program of China (grant no. 2018YFA0108300) and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (grant nos. 2019GXNSFBA245015 and 2020GXNSFAA297108).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HL, WC and ZQ designed the study. HL and DH analyzed the experimental data and wrote the manuscript. XT and YL assisted in the revision of the manuscript. HL, DH, XT, YL, QL, CL and HH performed the experiments. WC and ZQ designed the study, coordinated technical support and project funding. HL and DH confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The animal experiments and the protocols in the present study were approved by the Ethics Committee of Affiliated Hospital of Youjiang Medical University for Nationalities (Approval no. YYFY-LL-2020-17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 2013;12:941–966. doi: 10.1016/j.arr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: New insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke. 2015;10:143–152. doi: 10.1111/ijs.12434. [DOI] [PubMed] [Google Scholar]
- 3.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: Implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 5.Barber PA, Demchuk AM, Hirt L, Buchan AM. Biochemistry of ischemic stroke. Adv Neurol. 2003;92:151–164. [PubMed] [Google Scholar]
- 6.Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res. 2002;33:48–56. doi: 10.1034/j.1600-079X.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagata S. Apoptotic DNA fragmentation. Exp Cell Res. 2000;256:12–18. doi: 10.1006/excr.2000.4834. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Li J, Yang Y, Wang X, Zhang Z, Zhang L. Neuronal apoptosis in cerebral ischemia/reperfusion area following electrical stimulation of fastigial nucleus. Neural Regen Res. 2014;9:727–734. doi: 10.4103/1673-5374.131577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin KJ, Sun FY. Effect of dextromethorphan, a NMDA antagonist, on DNA repair in rat photochemical thrombotic cerebral ischemia. Brain Res. 1999;815:29–35. doi: 10.1016/S0006-8993(98)01071-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang Y, Jin XF, Zhou XH, Dong XH, Yu WT, Gao WJ. The role of astragaloside IV against cerebral ischemia/reperfusion injury: Suppression of apoptosis via promotion of P62-LC3-Autophagy. Molecules. 2019;24:1838. doi: 10.3390/molecules24091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Huang G, Chen S, Gou Y, Dong Z, Zhang X. Homocysteine aggravates cortical neural cell injury through neuronal autophagy overactivation following rat cerebral ischemia-reperfusion. Int J Mol Sci. 2016;17:1196. doi: 10.3390/ijms17081196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su J, Zhang T, Wang K, Zhu T, Li X. Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochem Res. 2014;39:2068–2077. doi: 10.1007/s11064-014-1396-x. [DOI] [PubMed] [Google Scholar]
- 15.Xu F, Fang Y, Yan L, Xu L, Zhang S, Cao Y, Xu L, Zhang X, Xie J, Jiang G, et al. Nuclear localization of Beclin 1 promotes radiation-induced DNA damage repair independent of autophagy. Sci Rep. 2017;7:45385. doi: 10.1038/srep45385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 17.Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, DC: 2011. [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Damia G. Targeting DNA-PK in cancer. Mutat Res. 2020;821:111692. doi: 10.1016/j.mrfmmm.2020.111692. [DOI] [PubMed] [Google Scholar]
- 21.Schmidley JW. Free radicals in central nervous system ischemia. Stroke. 1990;21:1086–1090. doi: 10.1161/01.STR.21.7.1086. [DOI] [PubMed] [Google Scholar]
- 22.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Zhao G, Zhang W, Li L, Wu S, Du G. Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules. 2014;19:15786–15798. doi: 10.3390/molecules191015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9:1321–1333. doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- 25.Xing S, Zhang Y, Li J, Zhang J, Li Y, Dang C, Li C, Fan Y, Yu J, Pei Z, Zeng J. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8:63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 26.Shi CX, Jin J, Wang XQ, Song T, Li GH, Li KZ, Ma JH. Sevoflurane attenuates brain damage through inhibiting autophagy and apoptosis in cerebral ischemia-reperfusion rats. Mol Med Rep. 2020;21:123–130. doi: 10.3892/mmr.2019.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Wang D, Zhang T, Lu X, Duan F, Ju L, Zhuang X, Jiang X. Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front Pharmacol. 2020;11:84. doi: 10.3389/fphar.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohatgi RA, Janusis J, Leonard D, Bellvé KD, Fogarty KE, Baehrecke EH, Corvera S, Shaw LM. Beclin 1 regulates growth factor receptor signaling in breast cancer. Oncogene. 2015;34:5352–5362. doi: 10.1038/onc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian M, Chen Y, Tian D, Qiao X, Ma Z, Li J. Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in gastric cancer cells. Gene. 2017;626:48–53. doi: 10.1016/j.gene.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Fremont S, Gerard A, Galloux M, Janvier K, Karess RE, Berlioz-Torrent C. Beclin-1 is required for chromosome congression and proper outer kinetochore assembly. EMBO Rep. 2013;14:364–372. doi: 10.1038/embor.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji B, Cheng B, Pan Y, Wang C, Chen J, Bai B. Neuroprotection of bradykinin/bradykinin B2 receptor system in cerebral ischemia. Biomed Pharmacother. 2017;94:1057–1063. doi: 10.1016/j.biopha.2017.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.