Abstract
Objectives
We evaluated the antibody response, natural killer cell response and B cell phenotypes in healthcare workers (HCW) who are vaccinated with two doses of CoronaVac with or without documented SARS-CoV-2 infection and unvaccinated HCWs with SARS-CoV-2 infection.
Methods
HCWs were divided into four groups: vaccine only (VO), vaccine after SARS-CoV-2 infection (VAI), SARS-CoV-2 infection only (IO), and SARS-CoV-2 infection after vaccine (IAV). Anti-SARS-CoV-2 spike protein (Anti-S) antibodies were measured by Elecsys Anti–SARS–CoV–2 S ELISA kit. Memory B cells (CD19+CD27+), plasmablast B cells (CD19+CD138+) and long-lived plasma cells (LLPC; CD138+CD19-) were measured by flow cytometry in 74 patients. Interferon gamma (IFN-γ) release by natural killer (NK) cells were measured by NKVue Test (NKMAX, Republic of Korea) in 76 patients. RT-PCR was performed with Bio-speedy® COVID-19 qPCR detection kit, Version 2 (Bioexen LTD, Istanbul, Turkey).
Results
The Anti-S antibodies were detectable in all HCWs (n: 224). The median Anti-S titers (BAU/mL) was significantly higher in VAI (620 25–75% 373–1341) compared to VO (136, 25–75% 85–283) and IO (111, 25–75% 54–413, p < 0.01). VAI group had significantly lower percentage of plasmablasts (2.9; 0–8.7) compared to VO (6.8; 3.5–12.0) and IO (9.9; 4.7–47.5, p < 0.01) (n:74). Percentage of LLPCs in groups VO, VAI and IO was similar. There was no difference of IFN-γ levels between the study groups (n: 76).
Conclusion
The antibody response was similar between uninfected vaccinated HCWs and unvaccinated HCWs who had natural infection. HCWs who had two doses of CoronaVac either before or after the natural SARS-CoV-2 infection elicited significantly higher antibody responses compared to uninfected vaccinated HCWs. The lower percentages of plasmablasts in the VAI group may indicate their migration to lymph nodes and initiation of the germinal center reaction phase. IFN-γ response did not differ among the groups.
Keywords: COVID-19, SARS-CoV-2 infection, Whole virion inactivated vaccine, CoronaVac, IFN-gamma, Anti-spike antibody
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak caused by a novel human coronavirus had started in late December 2019, which has later turned into a global pandemic [1]. Despite several public health precautions, the outbreak is still ongoing. As there are no antivirals to treat the SARS-CoV-2 infection, fast introduction of newly developed vaccines against SARS-CoV-2 has been the main global strategy to control the pandemic.
Turkey has started mass vaccination with an inactivated whole virion vaccine, CoronaVac (Sinovac Life Science Co, Ltd, Beijing, China) in January 2021 and priority was given to healthcare workers (HCWs). In this program, two doses of CoronaVac were administered intramuscularly 28 days apart, including individuals who previously had had SARS-CoV-2infection [2]. According to the European Centre for Disease Prevention and Control report; as of 11 June 2021, a total of 333,678,903 COVID-19 vaccine doses had been distributed by manufacturers to European Union/European Economic Area (EU/EEA) countries. The number of HCWs is 1.061.035 in Turkey and vaccination priority was given to HCWs in January by administering 2 doses of inactivated SARS-CoV-2 vaccine one month apart (CoronaVac). A study from Turkey reported reduced crude mortality rates among HCWs after CoronaVac (between 2021 April 1 – May 17) compared to the pre-vaccination period (between 2020 March-2021 January) [3].
Although published studies were limited about the efficacy of inactivated whole virion vaccines, numerous publications about mRNA vaccines emerged fast. Human body generates antibodies directed against the spike protein of the virus (Anti-S) following SARS-CoV-2 infection, which declines months after the infection [4], [5]. BNT162b2 mRNA COVID-19 (Pfizer/BioNTech) vaccine trials have shown 95% efficacy in preventing symptomatic infection in individuals without previous SARS-CoV-2 infection [6], [7], [8], [9]. Trials with existing vaccines have shown varying degrees of humoral and cellular immune responses against SARS-CoV-2 [10], [11], [12], [13], [14], [15], [16]. A recent study done among fully vaccinated HCWs showed that humoral immune response decreases substantially in six months after the administration of the second dose of BNT162b2 vaccine [17]. It was also shown that in patients with previous SARS-CoV-2 infection, the antibody responses were increased after BNT162b2 mRNA COVID-19 (Pfizer/BioNTech) and mRNA-1273 (Moderna) vaccines [18], [19]. The phase 3 trial results of CoronaVac from Turkey showed anti-receptor-binding domain (RBD) antibodies in 89.7% of vaccinated individuals’ serum samples taken 14 days after the second dose [16]. There is very limited data about immune responses to CoronaVac, and antibody responses to vaccination after previous natural infection [20], [21], [22]. One study from Turkey had showed augmented antibody response to CoronaVac in individuals with previous SARS-CoV-2 infection [23].
There are studies investigating the peripheral blood lymphocyte profiles and bone marrow cell response to infection. SARS-CoV-2 spike protein-binding memory B cells were present at significantly higher frequencies than healthy controls at least 7 months after the onset of symptoms [6]. Adequate serum antibody titers are maintained by long-lived plasma cells (LLPC), which are non-replicating, antigen-specific plasma cells that are detected in the bone marrow long after the antigen is cleared [24].
NK cell driven interferon response and its effect on prognosis of SARS-CoV-2 infection has also been investigated. Studies have showed that patients with critical or severe infection had lower type I interferon (IFN) response [25]. On the other hand, increased IFN response have also been reported in patients with severe infection [26]. It has also been reported that measuring IFN response could be an indicator of the activation of innate immune response [27].
We aimed to determine 1) the humoral antibody response in HCWs who were vaccinated with CoronaVac, HCWs who had natural infection and compare the magnitude of the humoral antibody response of HCWs who were vaccinated and had COVID-19 infection, 2) to determine whether vaccination and/or natural infection have any effect on B cell phenotype, and natural killer (NK) cell response parameters 3) to describe the frequency of adverse events in vaccinated HCWs.
2. Methods
2.1. Study setting
The study was conducted between March – June 2021 in Marmara University Pendik E&R Hospital, Istanbul, Turkey on volunteering HCWs. The study was approved by the ethical committee of Marmara University (09.2020.740) and Turkish Ministry of Health. All participants have provided written informed consent. Investigators collected demographic information and adverse events occurring within 14 days after vaccination.
The participants were divided into four groups as shown below and blood samples were drawn to determine the anti-S antibodies, immunophenotyping and NK activation by IFN- γ.
-
•
Vaccine only (VO); HCWs without documented SARS-CoV-2 infection and had received two doses of CoronaVac
-
•
Vaccine after SARS-CoV-2 infection (VAI); HCWs who had had confirmed SARS-CoV-2 infection previously and had received two doses of CoronaVac afterwards
-
•
SARS-CoV-2 infection only (IO); HCWs who had had confirmed SARS-CoV-2 infection previously and had not received CoronaVac
-
•
SARS-CoV-2 infection after vaccine (IAV); HCWs who had received two doses of CoronaVac and later had confirmed SARS-CoV-2 infection (≥2 weeks after the second dose of vaccination).
The adverse effects that might have occurred during the 14 days following each dose of vaccination was investigated by a questionnaire inquiring fever, myalgia, runny nose, fatigue, sore throat, rash, shortness of breath or other symptoms at the time blood samples were drawn. In addition, more serious adverse effects such as anaphylaxis and allergies occuring within a few hours after each dose of vaccination was sought via the hospital information system.
The groups were matched for age, gender, time after the SARS-CoV-2 infection and/or time after second dose of vaccine to blood sampling, except for IAV group, who were included later.
3. Laboratory studies
3.1. Antibody response
Antibodies (predominantly IgG, but also IgA and IgM) against the receptor binding domain (RBD) of the S protein of the SARS–CoV–2 virus were determined quantitatively in subjects’ sera with a double-antigen sandwich immunoassay with electrochemiluminescence detection (Elecsys Anti–SARS–CoV–2 S kit, Cobas-e 601 analyzer, Roche Diagnostics, Basel, Switzerland). The results were obtained as U/mL. Since the Roche Elecsys Anti - SARS-CoV-2 S units per mL and WHO International Standards for anti-SARS-CoV-2 immunoglobulins were closely correlated (r2 = 0.9992, slope = 0.972, intercept = 0.0072), the results were converted to BAU/mL (binding antibody units/mL) of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin [28], [29].
3.2. Isolation of white blood cells (WBC) and B cell immunophenotyping
Peripheral blood WBCs were isolated from whole blood samples by using erythrocyte lysing solution. The following combinations with fluorochrome labelled monoclonal antibodies (mAb) and isotype-matched controls were used for three colors phenotypic analysis: 1) CD45-FITC / CD138-PE / CD19-PerCP-Cy.5.5; 2) CD45-FITC / CD27-PE / CD19-PerCP-Cy.5.5 (Becton Dickinson Inc, San Jose, CA, USA). Following the incubation of 5x105 cells per tube with adequate amount of antibodies as recommended by manufacturer for 20 minutes at room temperature in the dark, cells were washed and were immediately acquired and then analyzed using Diva software on a FACSCanto II (Becton Dickinson Inc, San Jose, CA, USA). Debris was excluded by using a gate that included all WBCs in the forward and side scatter plot or by using CD45/SSC plot. Lymphocytes were gated according to their forward and side scatter characteristics. CD138+CD19+ (plasmocytoid B cells); CD138+CD19- (long lived plasma cells, LLPC); CD27+CD19+ (memory B cells) populations were evaluated as percentages.
3.3. NK activation by IFN-γ measurement
IFN-γ secretion by NK cells upon overnight activation by a specific NK activator was determined (NKVue Test, NKMAX, Republic of Korea). Briefly, peripheral venous blood samples were taken into special tubes which include NK stimulator (patented product) provided by the manufacturer. Tubes were directly incubated at 37 °C, 5% in a humidified CO2 incubator during night. Next day, tubes were centrifuged at 2000 rpm for 15 minutes. Then, IFN-γ levels were measured in the supernatants of the tubes by using an IFN-γ ELISA kit provided by the manufacturer. The reference values for the NKVue test are > 500 pg/mL for normal, between 200 and 500 pg/mL for borderline and < 200 pg/mL for low IFN-γ response.
3.4. RT_PCR for SARS-CoV-2
Viral RNA was extracted from respiratory samples by using Bio-speedy® viral nucleic acid buffer (Bioexen LTD, Istanbul, Turkey) and RT-PCR was performed with Bio-speedy® SARS CoV-2 RT- qPCR detection kit, Version 2 (Bioexen LTD, Istanbul, Turkey) using primers and probes targeting the nucleocapsid (N) and ORF-1ab gene regions found in all SARS-CoV-2 and Human RNase P gene for the routine screening in a Biorad CFX-96 System (Biorad Laboratories INC., California, United States) B.1.1.7 detection for SARS CoV-2B.1.1.7 (UK) variant: Bio-speedy® SARS CoV-2 + VOC202012/01 RT-qPCR kit V1.0 (Bioexen LTD, Istanbul, Turkey) were used.
3.5. Statistics:
Descriptive analyses were presented as frequency and percentages, continuous values were expressed as [median (25th-75th percentiles)]. Categorical variables were compared with chi-square or Fisher’s exact test. Paired proportions were compared with McNemar’s test. Continuous variables between groups were compared with Mann-Whitney U test.
The VAI group would have higher antibody levels when compared to VO and IO groups was the main hypothesis of the study. Although there is a fourth group (IAV), this group was not included in the main hypothesis testing since the time elapsed from vaccination to blood collection was not comparable to other groups. A secondary analysis was done comparing the VAI and IAV groups. The analyses were performed with SPSS software version 23.0.
4. Results
A total of 224 HCWs were included in the study. The characteristics of the study groups are presented in Table 1 . The median age was 31 (28–40) years and 56.4 % were female. The gender and age distribution were similar among groups. At least one comorbidity was present in 59 participants (26.3%). The most common comorbidity was asthma.
Table 1.
Descriptive characteristics of the study groups.
| VO (n = 75) | VAI (n = 53) | IO (n = 60) | IAV (n = 36) | |
|---|---|---|---|---|
| Age, median, (%25–75) | 35 (28–42) | 31 (29–41) | 31 (27–40) | 30 (26–39) |
| Gender, female, n (%) | 43 (57.3) | 30 (56.6) | 34 (48.6) | 25 (69.4) |
| Body mass index | 24 (22–27) | 25 (22–28) | 25 (23–28) | 24 (22–27) |
| Comorbidity present, n(%) | 16 (21.3) | 20 (37.7) | 14 (20.3) | 9 (25.0) |
| Days from COVID-19 diagnosis to blood collection | NA | 167 (135–333) | 162 (125–296) | 52 (48–59) |
| Days from second dose of vaccination to blood collection | 46 (41–47) | 44 (42–46) | NA | 109 (104–109) |
| Days from COVID-19 diagnosis to second dose of vaccination | 94 (62–261) | 55 (45–59) | ||
| Hospitalized, n (%) | NA | 8 (15.1) | 3 (5.0) | 0 |
| Comorbidity, n (%) | 16 (21.3) | 20 (37.7) | 14 (23.7) | 9 (25.0) |
| Asthma | 4 (5.3) | 2 (3.8) | 2 (3.3) | 4 (11.1) |
| Thyroid disorders | 2 (2.7) | 3 (5.7) | 4 (6.7) | 1 (2.8) |
| Allergic rhinitis | 0 | 7 (13.2) | 1 (1.7) | 1 (2.8) |
| Hypertension | 2 (2.7) | 3 (5.7) | 1 (1.7) | 1 (2.8) |
| Diabetes | 1 (1.3) | 2 (3.8) | 0 | 1 (2.8) |
| Malignancy | 2 (2.7) | 1 (1.9) | 1 (1.7) | 0 |
| Rheumatologic | 0 | 3 (5.7) | 0 | 1 (2.8) |
| Miscellaneous | 9 (12.0) | 0 | 5 (8.3) | 1 (2.8) |
| Adverse events | ||||
| Any adverse event after first or second dose | 23 (30.7) | 18 (34.0) | – | 12 (33.3) |
| Fatigue | 14 (18.7) | 1 (1.9) | – | 7 (19.4) |
| Myalgia | 8 (10.7) | 2 (3.8) | – | 4 (11.1) |
| Pain at injection site | 4 (5.3) | 3 (5.7) | – | 3 (8.3) |
| Sore throat | 4 (5.3) | 0 | – | 1 (2.8) |
| Fever | 0 | 1 (1.9) | – | 2 (5.6) |
| Dyspnea | 1 (1.3) | 1 (1.9) | – | 1 (2.8) |
| Rash | 0 | 1 (1.9) | – | 1 (2.8) |
| Other | 7 (9.3) | 9 (17.0) | – | 3 (8.3) |
Abbreviations: VO; Vaccine Only, VAI; Vaccine After Infection, IO; Infection Only, IAV; Infection After Vaccination, NA; not applicable.
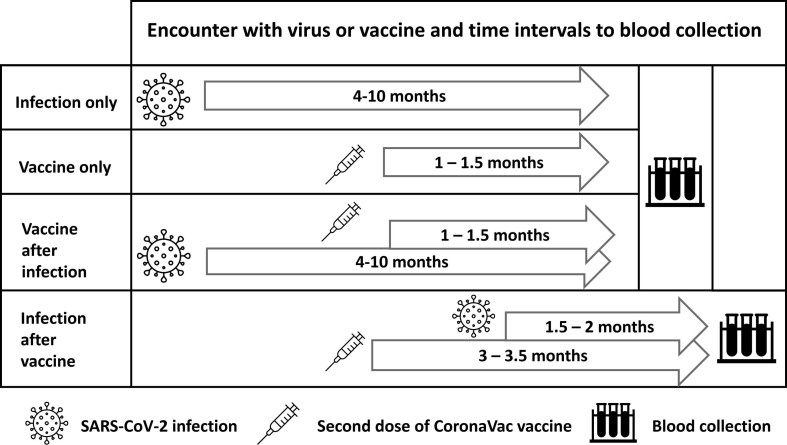
The median days from the second dose of vaccine to blood collection were 46 (41–47), 44 (42–46), and 109 (104–109) in groups VO, VAI, and IAV, respectively. The median days from COVID-19 diagnosis was 167 (135–333), 162 (125–296) and 52 (48–59) in groups VAI, IO, and IAV, respectively. A detailed explanation of intervals from infection and vaccination to blood collection is presented in Fig. 1 .
Fig. 1.
Diagram of intervals from infection and vaccination to blood collection.
The most common adverse events were fatigue (22/164), myalgia (14/164), and pain at injection site (10/164). The participants declared that the adverse events regressed within 48 h. Analysis of adverse events revealed that there was no significant difference among groups. VO group reported 23/75 (30.7%), VAI group had 18/53 (34.0%) and IAV group had 12/36 (33.3%) adverse events following any of the doses. Pairwise comparison showed significant decrease of adverse event frequency after the second dose (25.6% vs 17.7%, p = 0.04). The most common adverse events observed and the severity of all were similar to the findings in clinical phase trials.
4.1. Antibody response
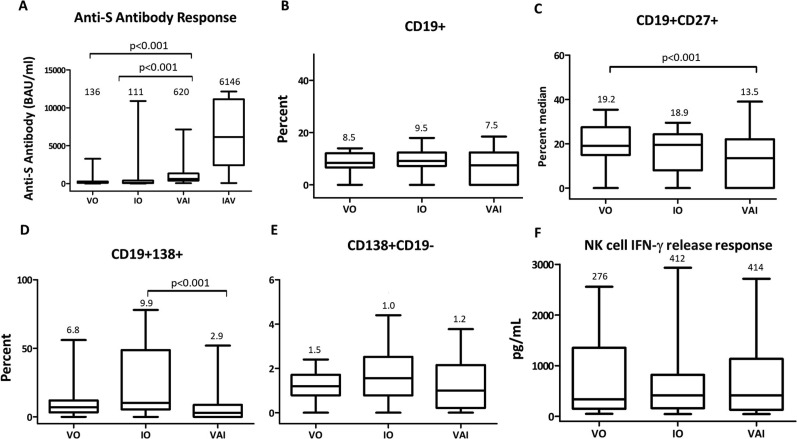
Anti-S antibody concentrations (BAU/mL) of VO (136; 85–283) and IO (111; 54–413) groups were comparably elevated and were not significantly different (p = 0.62) from each other, whereas, Anti-S antibodies were significantly higher in VAI group (620; 373–1341) compared to VO and IO groups (p < 0.01). Strikingly, the IAV group had the highest antibody levels (6146; 2426–11137), but this data was not compared statistically because the duration from the 2nd dose of vaccine to blood sampling did not match other groups’ intervals (Fig. 2 A).
Fig. 2.
Anti-S antibody concentrations (A), B cell percentages (B-E), and IFN- γ levels (F) measured in VO, IO, and VAI groups. Median values are noted above the box&whiskers plots. VO: Vaccine only, IO: infection only, VAI: infection after vaccination, IAV: infection after vaccination, NK: Natural killer, IFN- γ: interferon gamma.
4.2. Memory B cells, plasmablasts and long-lived plasma cells
B Lymphocyte percentages, memory B cells (CD19+CD27+), plasmablasts (CD19+CD138+) and LLPC (CD138+CD19-) were evaluated in a total of 74 patients across groups (27 in VO; 24 in VAI; 23 in IO). No difference was observed in the percentage of B cells (CD19+) among VAI (7.5; 0–12.4), VO (8.5; 7–12.0), and IO (9.3;7.3–12.0) groups (Fig. 2B).
VAI group had significantly lower percentage of CD19+138+ (2.9; 0–8.7) compared to VO (6.8; 3.5–12.0) and IO (9.9; 4.7–47.5) (Figure-2C) and lower percentage of CD19+27+ cells (13.5; 0–22.5) compared to VO (19.2; 15.0–26.6) and IO (18.9; 8.0–24.0) (p < 0.01) (Fig. 2D). There was no difference in the percentage of LLPC among groups (1.2 (0.7–1.7) in VO, 1.5 (07.-2.5) in IO and, 1.0 (0.2–2.1) in VAI groups) (Fig. 2E).
4.3. IFN- γ release by NK cells
NK cell response measured by IFN- γ release (pg/mL) upon stimulation was analyzed in 76 patients (9 in VO, 18 in VAI, 26 in IO and 23 in IAV group). The levels of interferon releasing response of NK cells of VO (276; 133–717), IO (412; 160–819), and VAI (414; 130–1105) groups were not different (Fig. 2F).
5. Discussion
We evaluated the anti-S antibody response in volunteering HCWs who were vaccinated with CoronaVac and had not documented SARS-CoV-2 infection until the time of blood sampling, HCWs who had had natural SARS-CoV-2 infection history before or after the vaccination and also HCWs who had natural SARS-CoV-2 infection history but were not vaccinated at the time of sampling in this observational, single-center case-control study. The anti-S antibody results in the VO group showed that the antibody response elicited with CoronaVac 4–6 weeks after the 2nd dose of vaccination was comparable to the antibody concentrations obtained after natural infection in 4–10 months’ time frame (25–75 percentile) in our IO cohort. Rus et al. had determined that a cut off of 133 BAU/mL for the Elecsys Anti–SARS–CoV–2 S kit to predict the presence of neutralizing antibodies [30]. In a recent article, one of the conclusions Gilbert et.al have reached was that the subgroups with neutralization titer 10 IU50/ml or with anti-spike IgG 33 BAU/ml, have about 75–85% reduction in COVID-19 risk compared to being unvaccinated [31]. Bergwerk et al. have showed that the neutralizing antibody titers were also correlated with IgG antibody titers [32], and higher antibody titers can provide better neutralizing activity which in the end results in better protection against infection [33], [34]. Both the VO and the IO groups’ anti-S antibody levels were high enough to assume the presence of neutralizing antibodies in our cohorts.
The VAI group, whose infection to sampling and vaccination to sampling times were matched to IO and VO groups respectively, had significantly higher anti-S antibody response, with a median of approximately 4–5 times than of IO and VO groups’. Similarly, Buonfrate et al had studied anti spike and anti- RBD IgG in 1935 HCWs and had reported that median antibody levels were significantly higher in individuals with past SARS-CoV-2 infection and were later vaccinated with Pfizer/Biontech [35]. Soysal et al. had investigated the immunogenicity of the CoronaVac in previously naturally infected HCWs and in line with our results, they showed that median antibody titers were significantly higher in previously infected HCWs (1220 AU/mL, range: 202–10328 AU/mL) compared to uninfected HCWs (913 AU/ml, range: 2.8–15547 AU/mL, p = 0.032). Yalçın et al, also has reported one log higher anti spike IgG levels in previously infected HCW’s after single dose of CoronaVac. The antibody levels were higher 28 days after the second dose of CoronaVac in previously infected HCWs compared to uninfected HCWs (mean 1280 AU/mL vs 899 AU/mL, p < 0.001) [36]. The high antibody levels in VAI group suggests that the CoronaVac can boost the antibody response primed by natural infection. Our finding was in-line with these researchers’ data showing that vaccination with either Pfizer/Biontech or CoronaVac vaccinations after natural infection exhibited a powerful booster effect and produced a significantly increased antibody response, which may be critical because Bergwerk et al have showed that HCWs with lower antibody titers are prone to re-infection with SARS-CoV-2 [32]. In several studies, previously infected HCWs elicited a higher antibody response compared to uninfected vaccinated and naturally infected individuals. Whether this difference translates to higher protection from infection needs further evaluation [9], [37], [38].
We observed that antibody levels of IAV group 6–8 weeks after the infection and 12 weeks after the vaccine was more than one log higher compared to VAI group (Fig. 2A). These two groups both had three encounters with the virus, either as vaccine or as natural infection. This group’s data couldn’t be statistically compared, since the times from the 2nd dose of vaccine to blood sampling were not matching other groups’ intervals, but nevertheless, the difference among levels were striking. The antibody response that increases rapidly a month after natural infection (or vaccination, similarly) starts to decrease slowly in the following months. The interval between first encounter with the virus (vaccination in IAV group) and blood sampling was much shorter than the VAI groups, which might at least partly be the cause for the high antibody concentrations in IAV group [17]. These findings suggest that encountering with the virus for a third time boosts the antibody response and the level of antibody response may depend on the interval between encounters.
We can suggest that either before or after infection, or without an encounter with Sars Cov−2, CoronaVac vaccine can induce considerable amount of antibody response. This data suggests that booster shots may not be necessary or can be delayed in people who are infected and vaccinated. The relatively lower antibody levels in IO and VO groups may suggest that priority should be given to those groups for a third booster dose. However, third booster administration decision for such patients should depend on robust controlled trial results [39].
Ekşioğlu-Demiralp et al. had previously shown that B lymphocyte counts and percentages were decreased in patients who had active COVID-19 [40]. We found that, unlike active infection, B lymphocyte (CD19+) percentages were all within normal limits with no difference among groups. This shows that the changes observed in B lymphocyte counts are a result of disease pathology, and the immunity acquired via natural infection or vaccination do not trigger related processes unless there is active disease.
One important marker in the assessment of humoral immunity evoked by either natural infections or through vaccination is the memory B (CD19+CD27+) lymphocytes [41]. The percentage of memory B cells evoked through vaccination (VO) or natural infection (IO) did not differ, and although the percentages observed in VAI group was found to be statistically lower than the VO group, all three groups were within refence ranges [42] . Since all three groups were within normal limits and the distribution of data in VAI group was wider, we did not attribute a clinical significance to the difference in the VAI group.
The plasmablasts as plasma cell precursors, and LLPC are responsible for antibody production. We observed the presence of plasmablasts (CD19+CD138+) in the peripheral blood in IO, VO, and VAI groups. The plasmablast percentages were comparable in IO and VO groups, but significantly decreased in the VAI group. The lower percentages of memory B cells and plasmablasts in the VAI group suggest that these cells might have migrated to lymph nodes from peripheral blood and have entered the germinal center reaction phase. Germinal center reaction is a very important process in humoral immunity where the proliferation of B lymphocytes and affinity maturation of immunoglobulins occur. The high antibody production in the VAI group supports this hypothesis [43].
Significantly higher percentage of plasmablasts in IO group is noticeable. There are studies which report that increased plasmablasts may be responsible from autoantibody production [44], [45] . The observance of autoantibodies in patients after COVID-19 have also shown to be associated with increased plasmablasts [46]. Therefore, the increase seen in IO group without an accompanying increase in anti S antibodies may be related with autoantibody production.
It was shown that SARS-CoV-2 infection had induced LLPC’s in a recent article, by enriching bone marrow derived plasma cells and testing their anti-S antibody production response [6]. We also detected the presence of LLPC’s in the peripheral blood in IO, VO and VAI groups, with no significant difference among groups. The presence of LLPC’s might be an indication of a sustained adaptive activation following at least 6 weeks after vaccination or natural infection, and the presence of a similar response in IO and VO groups and a more than 5-fold response in VAI group shows that SARS Cov-2 specific LLPC’s have been induced.
We found that IFN-γ release by NK cells upon stimulation was not different in all three groups, although the IFN-γ release data in each group was widely dispersed with the presence of very high and very low responders. NK activation is critical for viral defense due to their cellular cytotoxic function and their cytokine release in particular IFN-γ that activates both B and T cells [47]. It had been suggested that during COVID-19, SARS-COV2 was inhibiting the interferon signaling pathway [48]. Since we measured normal IFN-γ levels in all groups at least one month after infection, we might think that inhibitory effect of SARS-Cov2 on interferon secretion may be short lived, maybe encompassing only the active infection period.
5.1. Adverse effects
The frequencies of adverse effects reported after vaccinations were higher than the phase 3 clinical trials in Turkey (18.9%) [16], but were similar to the findings of another study in Turkey [49] (37.2% and 28.7%). The adverse event rates in VO, IAV and VAI groups were 30.7%, 33.3% and34.0%, respectively. Soysal et al. also reported that frequency of adverse events did not differ between the previously infected (35%) and uninfected (34%) groups [23]. The present study found similar adverse event rates without significant difference between the infected and uninfected groups. We also showed that the reporting of adverse reactions was significantly lower after the second dose of vaccination.
5.2. Strengths
To the best of our knowledge, this is the first study to investigate the humoral antibody response, B cell immunophenotype profile and NK cell response of individuals vaccinated with inactivated SARS-CoV-2 vaccine (CoronaVac) alone and together with natural SARS-CoV-2 infection.
5.3. Limitations
This study was conducted in healthcare workers, who were relatively young and healthy. This limits the generalizability of the study results for high-risk populations. The small sample size and lack of prevaccination antibody titers of the participants are other limitations.
6. Conclusion
The antibody response in uninfected vaccinated HCWs and HCWs who were unvaccinated and had had natural infection is similar to each other and comparable to levels obtained where the presence of neutralizing antibodies were shown. HCWs who had two doses of CoronaVac after natural SARS-CoV-2 infection elicited significantly higher antibody response, up to five times of the VO and IO groups’, proving the basis for the requirement of a third dose of vaccination. HCWs who had SARS-CoV-2 after two doses of vaccination had one log higher antibody response, all showing that a third encounter with the virus after immunization, boosted the antibody response significantly. All groups had similar B lymphocyte percentages but the lower plasmablast percentages in VAI group indicate the migration of antibody producing cells from plasma to lymph nodes. The presence of LLPCs in all groups with similar percentages is indicative of sustained adaptive activation after immunization modeled in our groups. Since none of the groups had active infection at the time of sampling, IFN-γ response did not differ among groups.
-
•
Author contributions
LM, GH, OS, AKY, SSY, MM, HB and EED initiated and coordinated the research. LM, GH, OS, EED, AKY, HB, MM, SSY, planned and recruited the study. LM, OS, GH, EED, AKY, US, HB, SSY and MM: conception and design of the study. OS, GH, AKY, MM, SSY, RCS and BC: acquisition of data. HB: statistical analysis. LM, OS, EED, AKY, US, MM, RCS, BC, SSY and HB: interpretation of data, drafting the article and revising it critically for important intellectual content. LM, OS, EED, GH, AKY, US, HB: final approval of the version to be submitted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N.A., Zhang D., Wang W., Li X., Yang B.O., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Turkish Ministry of Health Covid19 information page, https://hsgm.saglik.gov.tr/en [accessed 20 March 2022].
- 3.Akpolat T., Uzun O. Reduced mortality rate after coronavac vaccine among healthcare workers. J Infect. 2021;83(2):e20–e21. doi: 10.1016/j.jinf.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29(6):917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dan J.M., Mateus J., Kato Y.u., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science:abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 7.Li K., Huang B., Wu M., Zhong A., Li L.u., Cai Y., et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhards C., Thiaucourt M., Kittel M., Becker C., Ast V., Hetjens M., et al. Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection. Int J Infect Dis. 2021;107:221–227. doi: 10.1016/j.ijid.2021.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L’Huillier AG, Meyer B, Andrey DO, Arm-Vernez I, Baggio S, Didierlaurent A, et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clinical Microbiology and Infection 2021;27:784. e1-784. e8. 10.1016/j.cmi.2021.01.005 [DOI] [PMC free article] [PubMed]
- 10.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Shen H., Huang R., Tong X., Wu C. Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect Dis. 2021;21(8):1071–1072. doi: 10.1016/S1473-3099(21)00287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. MedRxiv. 2020 [Google Scholar]
- 16.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. The Lancet. 2021 doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muena N.A., García-Salum T., Pardo-Roa C., Serrano E.F., Levican J., Avendaño M.J., et al. Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines. MedRxiv. 2021 [Google Scholar]
- 21.Bayram A., Demirbakan H., Günel Karadeniz P., Erdoğan M., Koçer I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in health care workers. J Med Virol. 2021;93(9):5560–5567. doi: 10.1002/jmv.27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyahi E., Bakhdiyarli G., Oztas M., Kuskucu M.A., Tok Y., Sut N., et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41(8):1429–1440. doi: 10.1007/s00296-021-04910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soysal A., Gönüllü E., Karabayır N., Alan S., Atıcı S., Yıldız İ., et al. Comparison of immunogenicity and reactogenicity of inactivated SARS-CoV-2 vaccine (CoronaVac) in previously SARS-CoV-2 infected and uninfected health care workers. Human Vacc Immunotherapeut. 2021;17(11):3876–3880. doi: 10.1080/21645515.2021.1953344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halliley J., Tipton C., Liesveld J., Rosenberg A., Darce J., Gregoretti I., et al. Long-lived plasma cells are contained within the CD19− CD38hiCD138+ subset in human bone marrow. Immunity. 2015;43(1):132–145. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johum S, Degen H-J Correlation of the units (U) of the Elecsys® Anti-SARS-CoV-2 S assay to the “binding antibody units. of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Memo from Department of Research & Development for Centralized and Point of Care Solutions, Roche Diagnostics GmbH, Nonnenwald 2, 82377 Penzberg, Germany; 2021.
- 30.Resman Rus K., Korva M., Knap N., Avšič Županc T., Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune Assays Team § Moderna, Inc., Team § Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team § United States Government (USG)/CoVPN Biostatistics Team § Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Criscuolo E., Diotti R.A., Strollo M., Rolla S., Ambrosi A., Locatelli M., et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol. 2021;93(4):2160–2167. doi: 10.1002/jmv.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lustig Y., Sapir E, Regev-Yochay G., Cohen C., Fluss R., Olmer L., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. The Lancet Respiratory Medicine. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buonfrate D., Piubelli C., Gobbi F., Martini D., Bertoli G., Ursini T., et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: a prospective study. Clin Microbiol Infect. 2021;27(12):1845–1850. doi: 10.1016/j.cmi.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yalçın T.Y., Topçu D.İ., Doğan Ö., Aydın S., Sarı N., Erol Ç., et al. Immunogenicity After Two Doses of Inactivated Virus Vaccine in Healthcare Workers with and without Previous COVID-19 Infection: Prospective Observational Study. J Med Virol. 2022;94(1):279–286. doi: 10.1002/jmv.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 38.Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 39.Krause P.R., Fleming T.R., Peto R., Longini I.M., Figueroa J.P., Sterne J.A.C., et al. Considerations in boosting COVID-19 vaccine immune responses. The Lancet. 2021;398(10308):1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eksioglu-Demiralp E., Alan S., Sili U., Bakan D., Ocak I., Yurekli R., et al. Peripheral innate and adaptive immune cells during COVID-19: Functional neutrophils, pro-inflammatory monocytes and half-dead lymphocytes. MedRxiv. 2020 doi: 10.1002/cyto.b.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemori T., Tarlinton D., Hiepe F., Andreas R. Academic Press; Molecular Biology of B cells: 2014. B cell memory and Plasma cell development; pp. 227–249. [Google Scholar]
- 42.Caraux A., Klein B., Paiva B., Bret C., Schmitz A., Fuhler G.M., et al. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica. 2010;95(6):1016–1020. doi: 10.3324/haematol.2009.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oropallo M.A., Cerutti A. Germinal center reaction: antigen affinity and presentation explain it all. Trends Immunol. 2014;35(7):287–289. doi: 10.1016/j.it.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tipton C.M., Fucile C.F., Darce J., Chida A., Ichikawa T., Gregoretti I., et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–765. doi: 10.1038/ni.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenks S.A., Cashman K.S., Woodruff M.C., Lee F.-E.-H., Sanz I. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288:136–148. doi: 10.1111/imr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultheiß C., Paschold L., Willscher E., Simnica D., Wöstemeier A., Muscate F., et al. Maturation trajectories and transcriptional landscape of plasmablasts and autoreactive B cells in COVID-19. Iscience. 2021:103325. doi: 10.1016/j.isci.2021.103325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul WE. Fundamental Immunology. Fundamental Immunology. 7th ed., Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013, p. 1283.
- 48.Park A., Iwasaki A. Type I and type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host & Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gümüş H.H., Ödemiş İ., Alışka H.E., Karslı A., Kara S., Özkale M., et al. Side effects and antibody response of an inactive severe acute respiratory syndrome coronavirus 2 vaccine among health care workers. Revista Da Associação Médica Brasileira. 2021;67(12):1825–1831. doi: 10.1590/1806-9282.20210755. [DOI] [PubMed] [Google Scholar]