Figure 1. Targeting gene amplification in cancer via triplex formation.
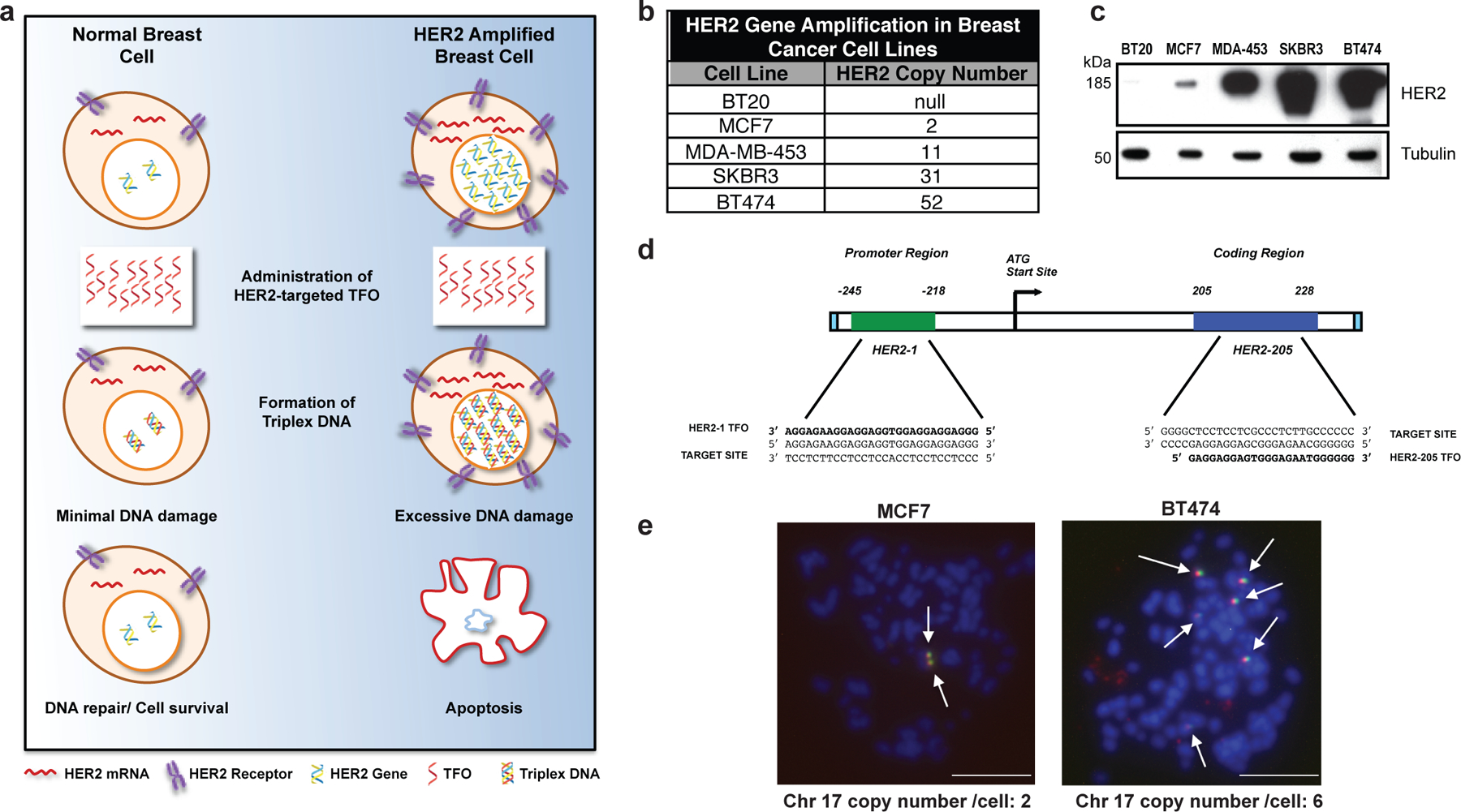
(a) Drug design scheme. Targeting the HER2 gene on a genomic level using DNA-binding molecules provides a therapeutic option to directly manipulate the DNA damage response pathways to specifically attack the HER2-amplified tumor. Triplex-induced DNA damage will only provoke apoptosis when multiple triplex structures are formed, while NER-dependent repair prevails in the presence of one or two structures. (b) Gene copy number characteristics of breast cancer cells lines 20. (c) Western blot analysis of HER2 protein levels in breast cancer cell lines with varying gene copy number. (d) TFOs bind as third strands in a sequence-specific manner within the major groove of duplex DNA at polypurine stretches. The specificity of these molecules arises from the formation of base triplets via reverse Hoogsteen hydrogen bonds between the third strand and the purine strand of the duplex DNA. Triplex structures were created in our studies using TFOs, HER2–1 and HER2–205 designed to bind to a polypurine sequence located either in the promoter or coding region of the HER2 gene. (e) Non-denatured metaphase chromosome spreads of MCF7 and BT474 breast cancer cells demonstrate chromosomal (blue) binding of TAMRA-HER2–205 (red) to its target site located on chromosome (chr.) 17 (green) (scale bar, 10μm).
