Figure 3. Factors affecting LRRC26 regulatory effects on BK G/V curves.
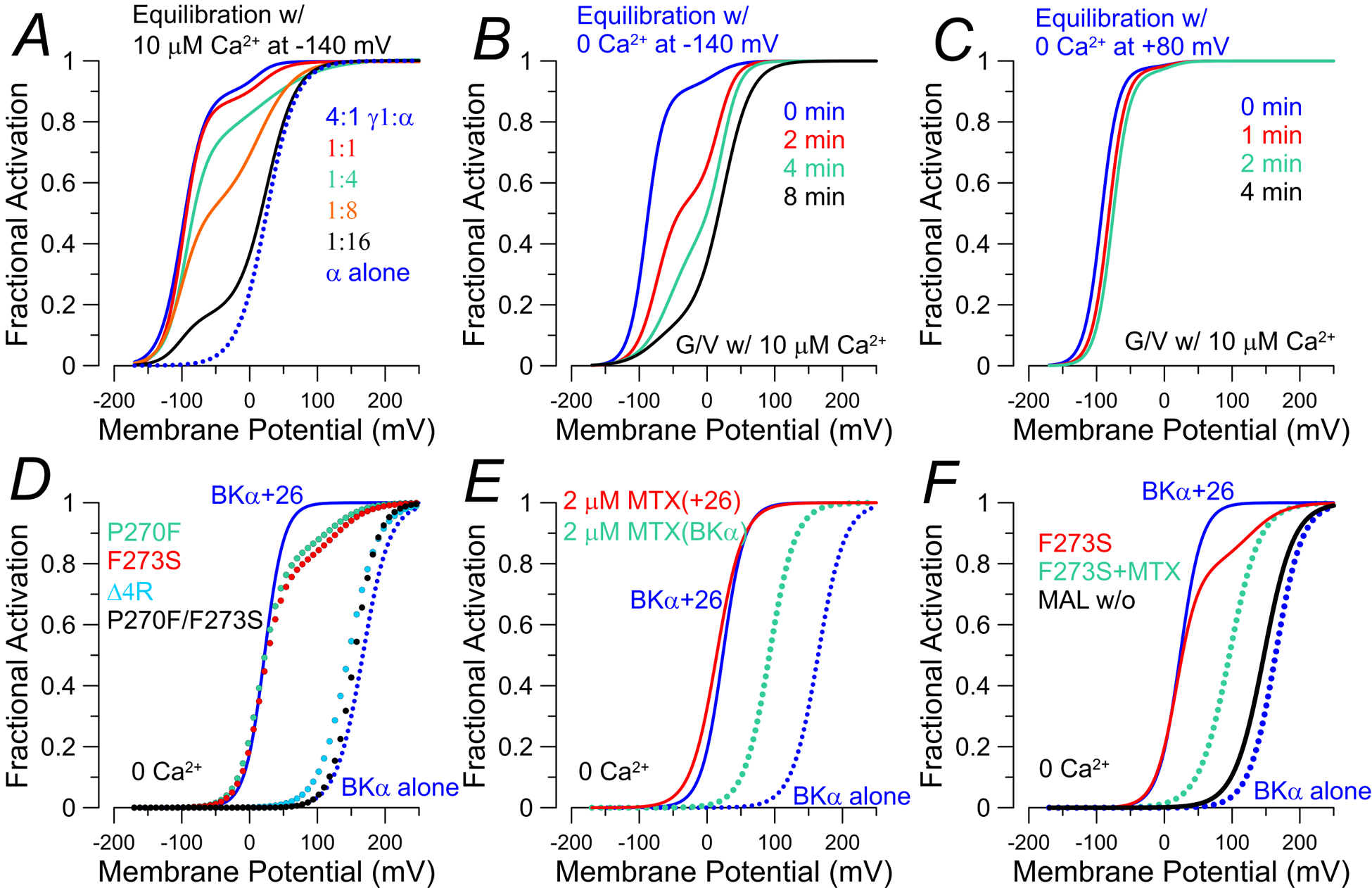
G/V curves were constructed from published values in the cited papers. For panels A-C, G/V curves were generated with 10 μM. A, BK G/V curves show loss of shifted (LVA) fraction as injected ratio of LRRC26:BKα is reduced from 4:1 to BKα alone (Gonzalez-Perez et al., 2014). B, for BK+LRRC26 channels expressed in oocytes, exposure of patches to 0 Ca2+ for the indicated times between acquisition of G/Vs with 10 μM Ca2+ results in gradual loss of shifted fraction of channels (Gonzalez-Perez et al., 2014). C, LVA behavior of BK+LRRC26 channels does not disappear if channels are held under conditions that promote channel activation (0 Ca2+, +80 mV, or 10 μM Ca2+, 0 mV) (Gonzalez-Perez et al., 2014). In D-F, G/V curves were generated with 0 Ca2+ with expression in HEK cells (solid blue lines: BKα+LRRC26; dotted blue: BKα alone). D, G/V curves for mutations in LRRC26 S6 TM segment that result in reductions or complete loss of LVA component of BK gating (Li et al., 2016). P270F and F273S result in loss of roughly over 80% of Co-IP with BKα, while Δ4R produces minimal effect on assembly with BKα, E, The BK activator, mallotoxin (MTX), shifts gating of BKα alone channels (dotted cyan), but not BKα+LRRC26 channels (dotted red) (Guan et al., 2017). F, for LRRC26-F273S (red line), following application of MTX the LVA component of BK current is abolished (dotted cyan), while still exhibiting a shift comparable to effect of MTX on BKα alone. Following washout of MTX (dotted red), the G/V becomes like BKα alone (Guan et al., 2017).
