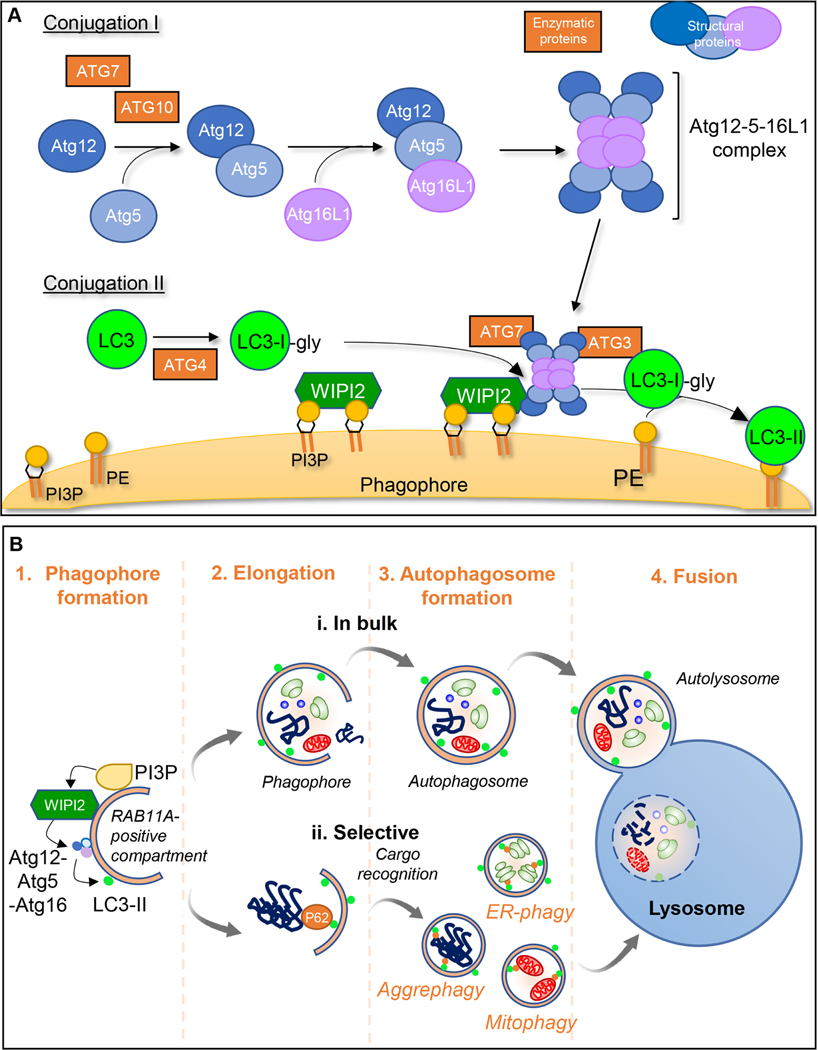
Figure 1. Schematic representation of macroautophagy:
Cell components to be degraded are engulfed in a double membraned structure called the phagophore, the edges of which elongate and close to form autophagosomes. These ultimately fuse with the lysosomal membrane for cargo degradation. A. Early steps in macroautophagy involve 2 ubiquitin-like conjugation cascades. Conjugation I leads to the formation of the Atg5-Atg12 conjugate mediated by ATG7 (E1-like) and ATG10 (E2-like). This then forms a complex with ATG16L1. During Conjugation II, ATG4 cleaves the C-terminus of LC3 generating LC3-I whose C-terminal glycine can be conjugated to phosphatidylethanolamine (PE) by ATG7 (E1-like), ATG3 (E2-like) and ATG5-ATG12/ATG16L1 (E3-like) (generating lipidated LC3 (LC3-II)). The other ATG8 family members (GABARAPs) use the same machinery to enable their conjugation to PE as LC3 proteins. The sites of LC3 conjugation to membranes are determined by the ATG5-ATG12-ATG16L1 complex, which localizes to the surface of the forming phagophore by interacting with WIPI2. WIPI2 is recruited to these membranes by binding both to phosphatidylinositol 3-phosphate (PI3P) and RAB11A. B. During autophagosome formation, the phagophore double membrane elongates and fuses to form a double-membraned vesicle termed the autophagosome. Cargo within autophagosomes can be trapped in a (i) bulk or (ii) selective manner by autophagy cargo receptors, such as P62, leading to the selective autophagy of specific substrates. Completion of vesicle closure to engulf regions of cytoplasm and organelles or to engulf specific cargoes such as aggregates (aggrephagy), mitochondria (mitophagy) or ribosomes (ribophagy) is followed by release from the recycling endosome-RAB11A platform to which the LC3 conjugates. Finally, the autophagosome outer membrane fuses with the lysosomal membrane and cargo is released for complete degradation in the lysosomal lumen.