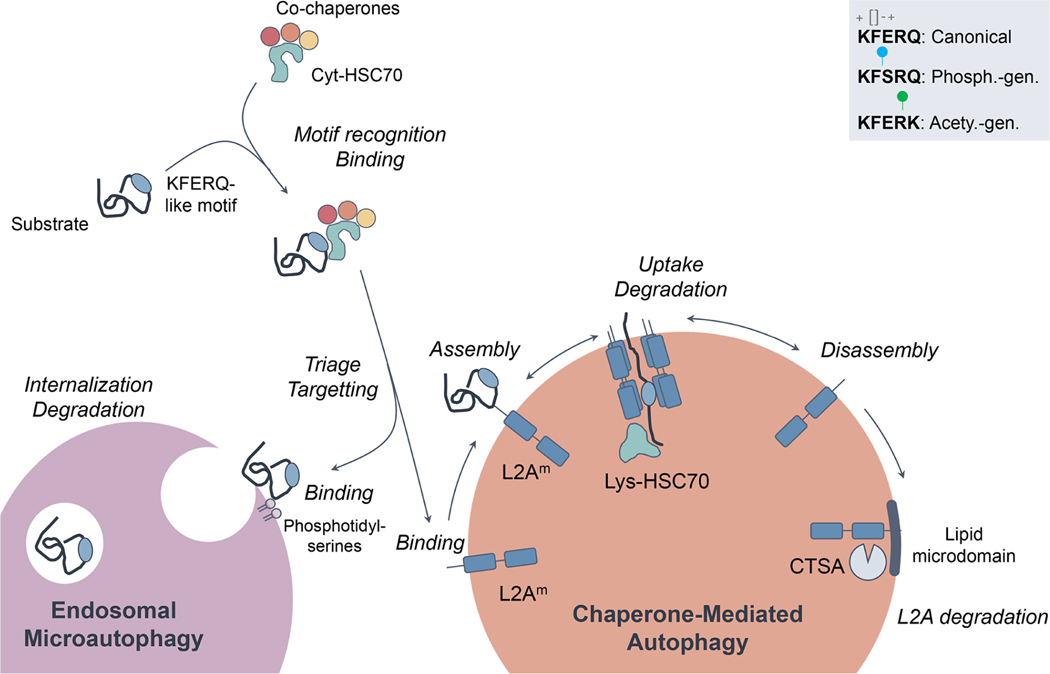
Figure 2. Schematic representation of chaperone-mediated autophagy (CMA) and endosomal microautophagy (eMI).
The first steps for cargo recognition are shared by CMA and eMI and are mediated by the binding of HSC70 to a targeting motif in the protein sequence biochemically related to the pentapeptide KFERQ. The inset (top right) highlights the chemical requirements for KFERQ motifs and the post-translational modifications such as phosphorylation or acetylation that can generate motifs by providing the missing charges. This motif is necessary and sufficient for CMA, whereas it is necessary but insufficient for eMI. Additional, as yet unknown, mediators are required for eMI targeting. HSC70 binds to the surface of late endosomes via phosphoserine and triggers assembly of the ESCRT machinery for internalization of substrate into intraluminal vesicles. In CMA, binding of the HSC70/substrate complex to LAMP2A at the lysosomal membrane triggers its multimerization to form a translocation complex that mediates the internalization of the substrate protein into the lumen for degradation. LAMP2A is actively disassembled from the complex to initiate a new cycle of binding/internalization. LAMP2A also mobilizes laterally to incorporate into lipid microdomains for its own degradation triggered by cathepsin A (CTSA). Phosph. gen.: phosphorylation generated. Acetyl. gen: Acetylation generated.