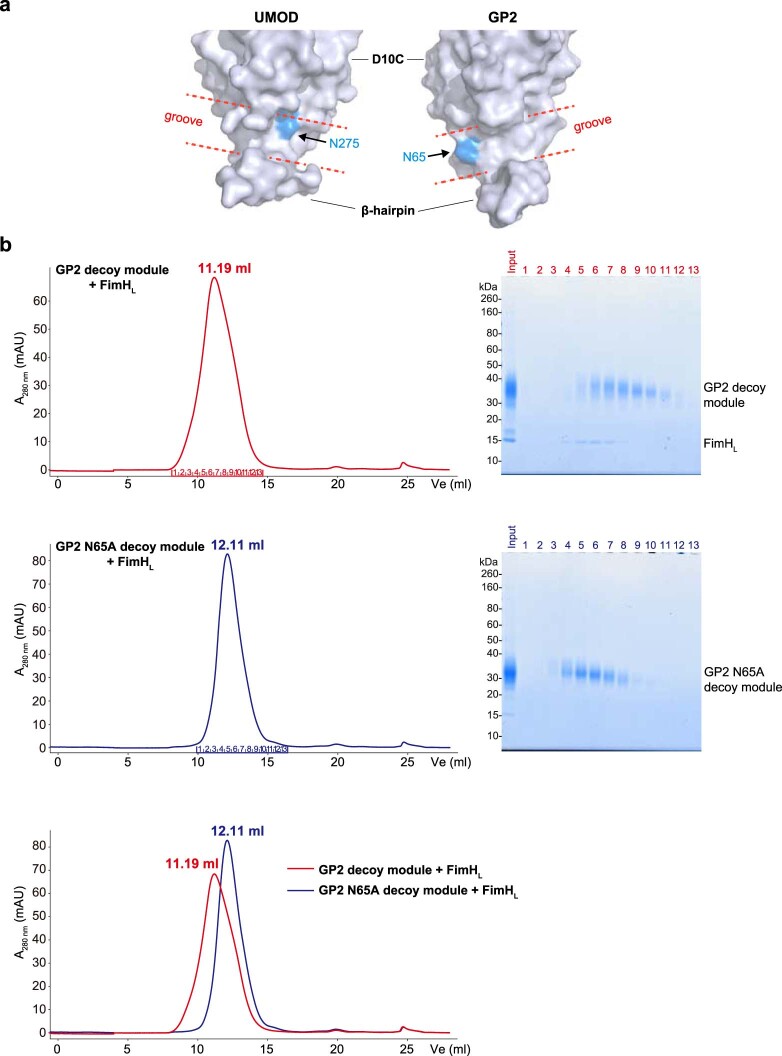
Extended Data Fig. 7. Inactivation of the N65 glycosylation site of GP2 impairs the interaction between the protein’s decoy module and FimHL.
a, The FimH-binding high-mannose glycan attached to UMOD N275 is located in the groove between the β-hairpin and D10C domain moieties of the protein’s decoy module (left panel). Although this sequon is not conserved in the decoy module of GP2, the groove of the latter contains a different, but closely spaced, N-glycosylation site at position 65 (right panel). b, SEC analysis of the material eluted after incubating an E. coli periplasmic extract containing untagged FimHL with wild-type or N65A mutant GP2 decoy modules immobilized on IMAC beads (left panels). Reducing SDS-PAGE analysis of the corresponding peak fractions (right panels) shows that FimHL binds to the wild-type GP2 decoy module but not to the N65A mutant. n = 2.