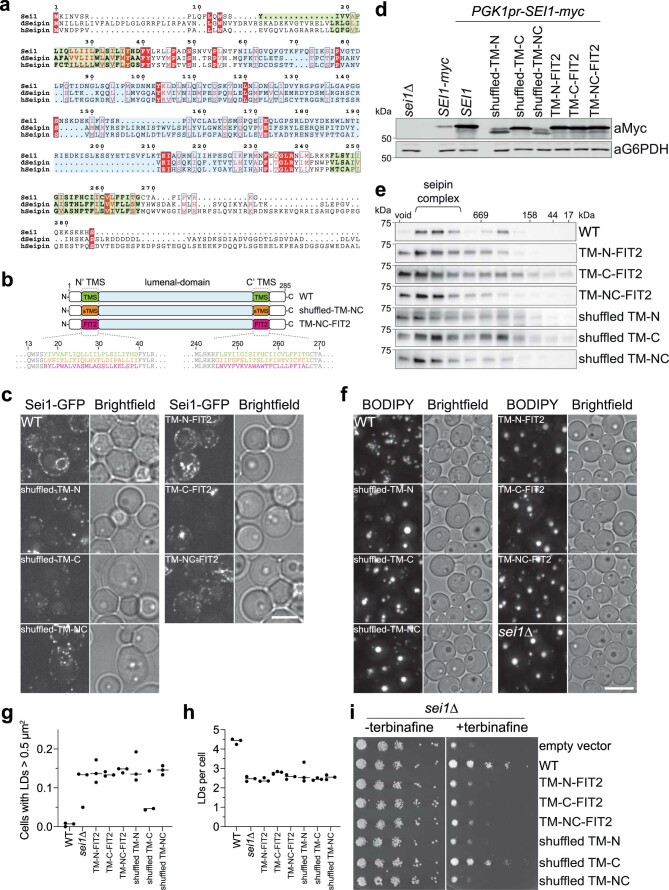
Extended Data Fig. 1. Transmembrane segments of seipin are conserved and required for function.
(a) Sequence alignment of yeast (Saccharomyces cerevisiae) seipin (Sei1) protein sequence with Drosophila melanogaster (dseipin) and human seipin (hseipin) in T-COFFEE55, plotted in ESPript 3.056. Identical residues are colored in red boxes, red characters and blue framed residues indicate similarity in a group or across groups, respectively. TM segments are colored in green and lumenal domains in cyan background similar to overview in b. (b) Overview of mutants analyzed in this figure. Detailed sequence information yeast seipin constructs is shown at the bottom for WT (green), shuffled-TMS (orange) and FIT2-TMS (pink). TMS, transmembrane segment. (c) Localization of seipin WT and mutant constructs expressed from plasmids in sei1∆ cells. Size bar = 5 µm. (d) Expression level of WT and mutant constructs tagged with C-terminal 13xmyc. SEI1-myc indicates expression level from endogenous promoter. (e) Transmembrane mutants form normal oligomers in detergent extracts. Size-exclusion analysis of membrane extract from cells expressing SEI1-13xmyc or indicated mutants from the endogenous locus driven by integrated PGK1 promoter. Representative immunoblots of two biologically independent experiment repeats are shown. (f) Analysis of LD morphology using BODIPY staining. Seipin mutants with C-terminal 13xmyc tag were expressed from PGK1 promoter. Size bar = 5 µm. (g,h) Quantification of experiment in panel f. n=3 biologically independent experiments. (i) Growth of indicated mutants on synthetic medium ± 100 µg/ml terbinafine.