Abstract
Cell death plays a pivotal role in the maintenance of tissue homeostasis. Key players in the controlled induction of cell death are the Death Receptors (DR). CD95 is a prototypic DR activated by its cognate ligand CD95L triggering programmed cell death. As a consequence, alterations in the CD95/CD95L pathway have been involved in several disease conditions ranging from autoimmune diseases to inflammation and cancer. CD95L-induced cell death has multiple roles in the immune response since it constitutes one of the mechanisms by which cytotoxic lymphocytes kill their targets, but it is also involved in the process of turning off the immune response. Furthermore, beyond the canonical pro-death signals, CD95L, which can be membrane-bound or soluble, also induces non-apoptotic signaling that contributes to its tumor-promoting and pro-inflammatory roles. The intent of this review is to describe the role of CD95/CD95L in the pathophysiology of cancers, autoimmune diseases and chronic inflammation and to discuss recently patented and emerging therapeutic strategies that exploit/block the CD95/CD95L system in these diseases.
Subject terms: Drug development, Apoptosis
Introduction
The division, differentiation, and death of a cell are highly regulated events in every developing organism and, in the adult individual, the loss of single cells plays a primary role in the maintenance of tissue homeostasis. Cell death, considered as a physiological event, can be defined as a highly evolved and conserved cell elimination mechanism, which responds to homeostatic and morphogenetic stimuli. The cells have a genetically-encoded death program that is finely controlled at the transcriptional and post-transcriptional levels. The definition of “programmed or regulated cell death” (RCD) is appropriate for the description of this phenomenon. Amongst the different types of RCD [1], apoptosis remains the most studied. Two major apoptotic pathways have been described: the extrinsic pathway or Death Receptor (DR) pathway and the intrinsic or mitochondrial pathway, which are linked [2]. In both pathways, specific aspartyl cysteine proteases (caspases) are activated and cleave cellular substrates, ultimately leading to the disruption of multiple cellular processes and morphological changes, such as cell shrinkage or the formation of apoptotic bodies, typical of apoptosis. The crosstalk between the two apoptotic pathways is carried out by the fact that caspase-8, involved in the extrinsic pathway, is able to cleave BID, a Bcl-2 family protein involved in the intrinsic pathway, thus activating the latter after apoptotic stimulus via DR and eventually strengthening the apoptotic signal [3–5].
Molecular bases of apoptotic signaling
The intrinsic mitochondrial-mediated apoptotic pathway
The intrinsic or mitochondrial pathway can be triggered by a variety of cellular stressors (e.g DNA-damaging agents, nutrient deprivation, hypoxia) and is tightly controlled by pro- and anti-apoptotic members of the Bcl-2 family of proteins. These cellular stress primarily lead to the increased transcription and/or post-translational activation of pro-apoptotic members of the Bcl-2 family of proteins [6, 7]. The key event of this intrinsic RCD is the mitochondrial outer membrane permeabilization (MOMP) induced by the oligomerization of the pro-apoptotic effector members of this family (BAX, BAK, and in some cases BOK) at the MOM [8]. MOMP allows the release of several caspase activators, such as the cytochrome c, from the mitochondrial intermembrane space to the cytosol. Hence, understanding the molecular bases of the pore-forming capacity of the effectors and of the regulation of their activation is crucial [8–11]. In the cytosol, cytochrome c promotes the assembly of a caspase activation platform called the apoptosome that also includes caspase-9, the activation factor of apoptotic proteases-1 (Apaf-1) and dATP [12]. Indeed, in the absence of apoptotic stimuli, Apaf-1 exists in an inactive monomeric conformation while it undergoes heptameric oligomerisation upon binding to cytochrome c and dATP in apoptotic conditions [13]. The formation of the apoptosome triggers the activation of caspase-9 which in turn activates the effector caspases-3, -7 that drive cell demise [14, 15]. MOMP also promotes the release of anti-apoptotic factors, such as the second mitochondrial activators of caspase (Smac/Diablo) and Omi/HtrA2 (high temperature requirement A2) and endonuclease G (EndoG) [16]. The protein Smac [17, 18] interacts with the BIR2 and BIR3 domains of the X-linked inhibitor of apoptosis protein (XIAP), neutralizing the inhibitory effect of XIAP on caspases-3, 7, and 9 [19]. Omi/HtrA2 [20–23] is a serine protease which, once released into the cytosol, is also able to significantly increase the activity of caspases by inhibiting XIAP. Noteworthy, MOMP can also induce non-apoptotic cell death such as ferroptosis, necroptosis and pyroptosis as recently reviewed elsewhere [7, 24].
The extent of MOMP largely defines the propensity of a cell to die or survive upon cell stress. The availability and activity of the different Bcl-2 family members influences the cellular readiness or “priming status” for MOMP. This priming status can be determined through BH3-profiling [25, 26] that evaluates MOMP upon incubation of permeabilized cells with BH3 peptides mimicking the action of some pro-apoptotic members of the Bcl-2 family. This assay has mainly been used to predict the sensitivity of cancer cells to various chemotherapeutic agents (resistant cells usually display lower priming) and to interrogate the sensitivity of cancer cells to the increasing arsenal of BH3-mimetics (molecules mimicking the activity of some pro-apoptotic Bcl-2 family members). MOMP is not necessarily a complete process. Indeed, partial MOMP has been observed when apoptotic induction is weak (minority MOMP) or accompanied by caspase inhibition (incomplete MOMP). The ability of cells to retain some non-permeabilized mitochondria, ATP synthesis and to eliminate damaged mitochondria influences their propensity to survive upon incomplete MOMP. Indeed, the remaining intact mitochondria can repopulate the whole mitochondrial pool [27, 28]. In the case of minority MOMP, caspase activation is insufficient to drive death but can promote DNA damage and genomic instability [29]. In addition, several reports indicate that MOMP can initiate multiple inflammatory signaling, for example the cGAS/STING [30, 31] or the NF-κB pathways [32]. Thereby MOMP can impact on the cell and its microenvironment beyond its ability to promote cell death.
Taken together, it appears that further understanding the mechanisms dictating the extent of MOMP, its ability to induce various types of cell death as well as non-death pathways in different pathophysiological contexts (e.g., upon pathogen infection, during tumor progression, etc.) and in different cell types will be required to fully expand the therapeutic targeting of the mitochondrial pathway. For further considerations on this topic, we advise readers to explore the many recent reviews available [7, 24, 33].
CD95 and CD95L: main structural features
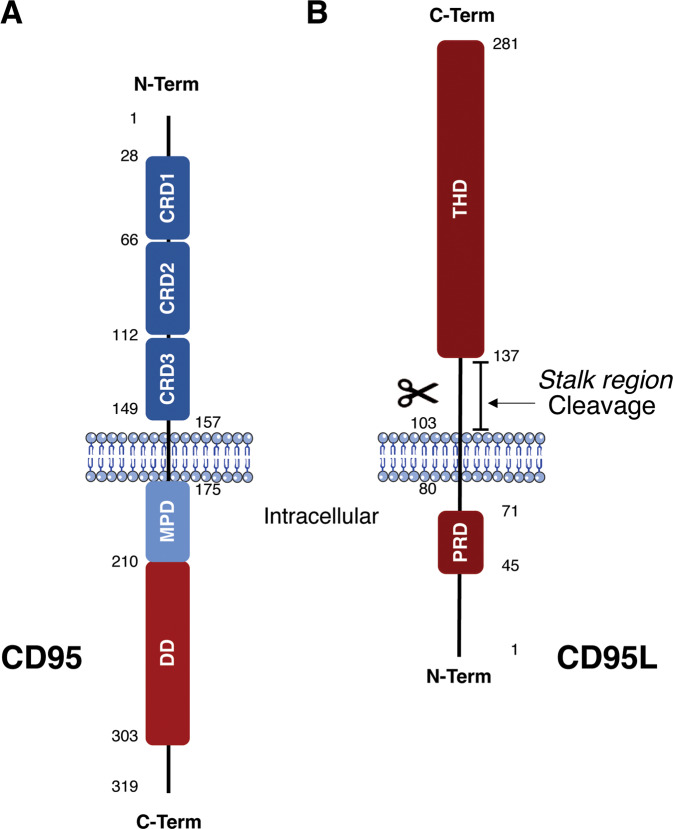
The extrinsic apoptosis pathway takes its name from the extracellular signal molecules that bind to receptors exposed on the surface of target cells, leading to a different way of activating the apoptotic signal compared to the mitochondrial-mediated one. There is a family of receptors specialized in the transmission of the signal upon binding by their cognate ligand that leads to the extrinsic programmed cell death: the DR. The DR belong to the Tumor Necrosis Factor Receptor (TNFR) superfamily, which counts a total of 29 receptors associated with a smaller selection of 19 ligands of the corresponding TNF ligands superfamily. CD95, TNFR1, DR3, DR4, DR5, and DR6 are the most studied DR that, upon ligand binding, convey death signal by using a conserved intracellular region of ~80 amino acids called the “Death Domain” (DD) [34]. This review particularly focuses on the DR CD95, its physiological ligand CD95L and the current approaches developed to therapeutically target this pair. CD95, encoded by the FAS gene, is a 319aa type I glycoprotein devoid of enzymatic activity that signals through protein-protein interaction. Mature CD95 is composed of three cysteine-rich extracellular domains, CRD3, CRD2, and CRD1 starting from the transmembrane domain and moving towards the N-Terminal. CRD2 and partly CRD3 are used for the recognition and binding of the ligand, while CRD1, comprising a subdomain called PLAD (Pre-Ligand Assembly Domain) [35, 36], is needed for the preassembly of CD95 in homodimeric or homotrimeric forms at the plasma membrane. The cytosolic region is composed of the previously mentioned Death Domain (DD) [34], which is essential for the transduction of the apoptotic signal, and a Membrane Proximal Domain (MPD) which conveys non-apoptotic signaling (Fig. 1) [37]. CD95L, encoded by the FASLG gene, consists of a total of 281aa, an extracellular region with a C-terminus and an intracellular region with an N-terminus. This protein is expressed at the plasma membrane in the form of a homotrimer thanks to the preassembly between monomers that takes place through an extracellular domain called TNF Homology Domain (THD) [38]. The THD also mediates receptor binding. The membrane-proximal extracellular stalk region is proteolytically processed by several metalloproteases to release soluble forms of CD95L (sCD95L), which generally display non-apoptotic activities (see part 2). The cytosolic region is then composed of an 80 amino acid tail containing a domain rich in proline, which is involved in the reverse signaling induced by CD95L–CD95 interaction in CD4 and CD8 T cells (Fig. 1). This reverse signaling involves the co-engagement of the TCR and co-stimulatory receptors along that of CD95/CD95L [39–42]. The reported outcomes of this reverse signaling depends on the cell type, with both proliferation and cell cycle arrest being reported, but the knowledge on this subject is still very partial.
Fig. 1. The CD95 receptor and its cognate ligand CD95L.
Schematic representation of the functional domains of the CD95 Death Receptor (A) and its ligand CD95L in its membrane-bound form (B). (DD Death Domain, MPD Membrane Proximal Domain, CRD Cysteine-Rich Domain, PRD Proline-Rich Domain, THD TNF Homology Domain).
Molecular bases of CD95-induced apoptotic signaling
CD95-mediated extrinsic apoptotic signaling begins with the binding of CD95L, via its THD on CRD2 and part of the CRD3 of CD95. In addition to the pre-association of CD95 mediated by the PLAD [35, 36], Fu et al. recently showed that proline motifs in the transmembrane (TM) domain also contribute to the trimerization of the receptor. Mutations of these motifs did not abrogate PLAD-mediated preassembly of unliganded CD95 but reduced CD95L-induced apoptosis, implying that these residues are important for stabilizing signaling-active CD95 oligomers [43]. Binding of CD95L has been proposed to trigger a reorganization of CD95 multimers and a conformational change in CD95 intracellular domain, allowing for the recruitment of the adaptor FADD (Fas-associated protein with Death Domain) to CD95 via DD-mediated homotypic interactions [44–47]. FADD is necessary for CD95L-induced apoptosis [48, 49]. In addition to its DD, FADD contains a Death Effector Domain and acts as a pivot for the assembly of DED filaments which are chains of proteins formed through DED-mediated interactions [50–52]. The DED chains nucleate from FADD [51–54] and also comprise procaspase-8 and cellular FLICE-like inhibitory proteins (c-FLIP) which are both key players in the cell death network [51, 52]. Extensive work has been undertaken, mainly in the past 15 years, to understand the mode of assembly of these structures. Beyond CD95- and TRAIL-R1/2-associated complexes, similar structures likely also nucleate from other death-inducing complexes such as the ripoptosome, inflammasomes, TNF-induced complex II, as well as the panoptosome [53, 55–57] and could thus influence cell fate upon a plethora of signals. In the case of CD95 signaling, the complex formed by CD95, FADD, caspase-8 and cFLIP constitutes a platform for caspase-8 activation which was first called the DISC (for Death-Inducing Signaling Complex) [44]. Procaspase-8 contains two DEDs, DED1, and DED2, located at its N-terminus and C-terminal large (p18) and small (p10) catalytic subunits. As described below, the formation of the DED filaments allows for the activation of caspase-8 which occurs via dimerization and a serie of internal cleavages, leading to the separation of the tandem DED from the catalytic subunits p18 and p10 [53, 54, 58–60]. The active p10 and p18 subunits are released into the cytoplasm to form mature active caspase-8 (Fig. 2). Fully matured caspase-8, an heterotetramer of two p18 and two p10, cleaves effector caspases-3, 6 and 7, which then cleave sub-cellular substrates, ultimately inducing cell death [61]. Three isoforms of cFLIP have been described: cFLIP long, short and related (cFLIPL, cFLIPS and cFLIPR). cFLIPS and cFLIPR comprise solely two tandem DED. In addition to the tandem DEDs, cFLIPL comprises a small and a large caspase-like catalytically inactive subunit. The initial DED-chain model, described by Inna Lavrik and Marion MacFarlane’s laboratories, proposed a nucleation of the chain from FADD involving an interaction between the DED of FADD with the DED1 of caspase-8, whilst further chain elongation implicated an interaction between the DED2 of FADD-associated caspase-8 with the DED1 of the incoming caspase-8, ultimately bringing the two catalytic domains of caspase-8 in close proximity [51, 52, 62, 63]. The molecular configuration of the DED filaments was further unveiled in 2016, by cryogenic electron microscopy (cryo-EM) analysis [53]. This study established that the orientation of the DED filaments actually relies on three different types of interactions (type I, II and III) between DEDs. Rather than a single linear chain nucleating from FADD through type I interactions, three strands of DED chains assemble via type II and III interactions to ultimately form a triple-helical structure [53, 54]. These different types of interactions define a hierarchy in the formation of the DED filaments, with FADD being rather poorly able to nucleate the DED of cFLIP, arguing against the theory of competition between procaspase-8 and c-FLIP for FADD. Thus, by affecting the conformation of caspase-8 and bringing in proximity the catalytic subunits of two procaspase-8, the DED-chain architecture works as a platform for the activation of this initiator caspase [64, 65].
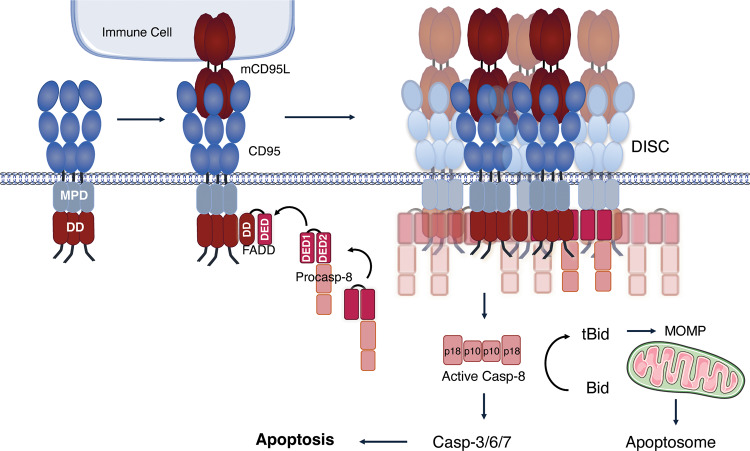
Fig. 2. CD95-dependent apoptotic signaling.
Representation of the CD95-mediated conventional or apoptotic pathway. The interaction between CD95 and its membrane-bound ligand mCD95L, triggers the recruitment of the adaptor protein FADD, which then recruits procaspase-8 generating the oligomerized DISC. The oligomerisation and auto-cleavage of procaspase-8 into its active form induces then the activation of the effector caspases-3, -6, -7 leading to apoptosis. Active caspase-8 is also able to cleave Bid, generating t-Bid that promotes Mitochondrial outer membrane permeabilization (MOMP) and thus the apoptosome-mediated effector caspase activation.
With regard to cFLIP proteins, it was first thought that these act by competing with caspase-8 for FADD binding or by preventing FADD self-association, akin to the viral FLIP MC159 [65], but this view has been challenged. Multiple evidence now demonstrate that cFLIPS/R actually precludes caspase-8 activation within the DISC. Indeed, reports highlighted that cFLIPS/R could limit DED-chain elongation and that cFLIPS/R incorporation into DED filaments actively prevented the formation of inter-strand assembly of caspase-8 catalytic domains [54, 63, 65]. Contrary to the small cFLIP isoforms, cFLIPL has been reported to possess a dual function, promoting or limiting caspase-8 activation and apoptosis. This is likely due to the fact that the cFLIPL/caspase-8 heterodimer does possess a catalytic activity, albeit DISC restricted, and that cFLIPL does not limit but promotes DED elongation. Hence, depending on the relative cellular amount of cFLIPL to caspase-8, cFLIPL might either facilitate the formation of filaments, and thereby of apoptosis-inducing caspase-8 homodimers (low cFLIPL to caspase-8 ratio) or, on the contrary (high cFLIPL to caspase-8 ratio), mainly result in formation of cFLIPL/caspase-8 heterodimers which, whilst able to cleave local substrates (e.g RIPK1), do not mediate apoptosis [63, 66–70].
Another initiator caspase, caspase-10, can be recruited to the TRAIL-R1/2 and CD95 DISC [66, 71, 72]. The role of this caspase in apoptosis induction by CD95L and TRAIL, and in particular its ability to substitute to caspase-8 loss, has been controversed. Caspase-10 is conserved in multiple other vertebrates [73] but lost in certain rodents (mice and rats) which has limited the study of its in vivo function. Some studies, mainly but not exclusively using Jurkat cells or primary T cells, reported that caspase-10 can contribute to DR-induced apoptosis, sometimes independently of caspase-8 [71, 74–79]. Interestingly, a recent study argued that this protease displays anti-apoptotic properties in certain cell lines [80]. Of note, this initiator caspase has been found as different splice variants in human cells, which have also been suggested to display opposing functions towards DR-mediated apoptosis [81]. How each of these isoforms and potentially their post-translational modifications (PTMs) impact on the DED-triple helix formation remains to be deciphered. Indeed, PTMs, most prominently glycosylation, phosphorylation and ubiquitination, of core components of the DISC proteins represent additional crucial checkpoints of DR signaling [82–84].
As mentioned above, caspase-8 also cleaves Bid, generating t-Bid that promotes MOMP and thus apoptosome-mediated effector caspase activation. Whether the engagement of the mitochondrial pathway downstream of CD95 is required for completion of apoptosis depends on the multiple variables described to influence DISC formation (e.g expression level of the DISC components, local lipid composition of the plasma membrane, etc.) as well as downstream regulators of the apoptosis pathway such as XIAP [85–87]. The discovery that caspase-8 is essential during embryonic development lead to the identification of its role as a regulator of necroptosis. Indeed, caspase-8, in concert with cFLIPL, is able to cleave RIPK1, along other key components of the necroptotic cascade, which limits necroptosis induction, as reviewed in [61]. In addition, as further developed later, several of the players of the apoptotic pathway, and in particular DISC components, are also involved in non-cytotoxic signaling outputs.
Involvement of CD95/CD95l in cancer and autoimmune diseases
Cancer
Multiple defects in the DR-mediated pathway have been observed in human tumors [88–91]. In healthy individuals, extrinsic apoptosis plays a central role in the immune-mediated elimination of infected or transformed cells. Therefore, defects in the extrinsic apoptotic pathway contribute to tumorigenesis primarily by limiting the efficiency of immune surveillance [92]. Cancer cells have different ways of escaping from apoptosis [93]. These include modification of the expression of pro- and anti-apoptotic proteins, such as inhibitors of apoptosis (IAPs) and the anti-apoptotic members of the Bcl-2 family among others, as well as the expression of CD95 itself at the membrane [94, 95]. Mutations in the FAS gene have been detected in both hematologic and solid tumor malignancies [96–99]. These mutations are mainly located in exon 8 and 9, which code for the DD, thus leading to resistance to CD95-mediated apoptosis [91, 93]. Accumulating evidence has shown that CD95 signaling cascades are often disrupted in several autoimmune diseases and malignant tumors [100–102], leading to the triggering of pro-tumorigenic cellular outcomes, rather than apoptosis [89, 103]. Considering the potential pro-tumorigenic effect of an incomplete induction of mitochondria-dependent death-signaling mentioned above, one could hypothesize that weak apoptotic signaling downstream of CD95 could also have tumor-promoting effects. Furthermore, the quality of cell death induced downstream of CD95 might also differentially impact on inflammation and tumor progression, even though this remains to be tested. In addition, several non-apoptotic pathways are also induced by CD95L, as detailed below, and contribute to its tumor-promoting and pro-inflammatory roles [88].
Non-apoptotic CD95-mediated pathways (NF-κB, MAPK, PI3K/Akt)
NF-κB pathway
Several studies reported that CD95-mediated stimulation can induce the apoptotic pathway in some cells, while in others, the non-apoptotic NF-κB (nuclear factor kappa B) pathway is favored [104, 105]. NF-κB is a transcription factor playing an important role in the inflammatory responses as well as in the regulation of cell survival, differentiation and proliferation. A non-optimal regulation of this signaling pathway has been associated with a high incidence of pathological conditions, such as cancer and chronic inflammation [106]. At the cell population level, the stimulation of CD95 by CD95L has long been reported to concomitantly induce apoptotic signaling and NF-κB activation [105, 107]. More recently, single cell studies have assessed if the apoptotic and NF-κB pathways were activated in the same cell [107, 108]. NF-κB was found to be activated in dying apoptotic cells, confirming the hypothesis that CD95-mediated NF-κB activation is correlated with the production of the so-called “find and eat me” pro-inflammatory cytokines, including IL-6, IL-8, CXCL1, MCP-1, and GMCSF [104]. Some of these cytokines act as chemokines and are therefore able to affect the tumor immune microenvironment.
Mechanistically, it appears that CD95 mediates NF-κB activation through a FADD and caspase-8-involving pathway [104, 109–111]. The Death Domain of CD95, FADD, and caspase-8 were in fact reported as required for NF-κB activation by CD95L [110]. Experiments carried out inhibiting caspases prevented TRAIL/anti-APO-1-induced apoptosis, but not NF-κB activation, indicating that both pathways bifurcate upstream of caspase-8 full activation [112]. Furthermore, the ability of DR to induce NF-κB activation was drastically reduced in a FADD-deficient CD95pos cell line (e.g., Jurkat cells) [112]. Caspase-8 participates in CD95L- and TRAIL-induced inflammatory signaling as a scaffold for assembly of a Caspase-8-FADD-RIPK1-containing complex, leading to NF-κB-dependent inflammation [109, 113]. Whilst this has not been studied for CD95 yet, it is tempting to speculate that NF-κB activation could also be ignited from the CD95 DISC, as recently shown for TRAIL [114]. Contrary to FADD and caspase-8 which seem to be essential for NF-KB activation upon CD95L, the role of RIPK1 in this process seems to be less pronounced and depends on the cell type [104, 109]. Recently, Horn et al. described a new role for caspase-10 that would negatively regulate the caspase-8-induced cell death, thus activating the cell survival induced by the NF-κB pathway [80]. TRADD, which is essential for the TNF-alpha-induced NF-κB activation, was not involved in the CD95L-induced NF-κB activation [110]. Experiments performed on cell lines resistant to CD95-mediated apoptosis, reported TRAF2 as a key player in pancreatic cancer pathophysiology [115]. This group also observed that the stimulation of TRAF2-overexpressing cells with CD95L led to induction of NF-κB, enhanced IL-8-secretion, and a further increased invasiveness. In fact, several E3 ligases contribute to NF-κB activation upon CD95 stimulation, namely cIAP1/2 and the Linear UBiquitin chain Assembly Complex (LUBAC), likely in a manner similar to their roles in TNF and TRAIL-induced gene-activation [104, 114, 116]. Downstream of these different actors, the activation of NF-κB relies on IκBα degradation, the protein responsible for constitutively inhibiting NF-κB. In a manner similar to TNF and TRAIL signaling, it is likely that several components of the CD95 DISC and/or secondary complex modified with ubiquitin allow the recruitment and activation of the IKK complex and potentially the TAB/TAK1 complex. The IKK complex is composed of three subunits (i.e., IKKα, IKKβ, IKKγ). The IKKβ subunit can then phosphorylate IκBα, marking it for lysine-48 ubiquitination and degradation by the proteasome. This leads to the translocation of NF-κB into the nucleus which promotes the expression of multiple genes including pro-inflammatory cytokines as well as anti-apoptotic proteins, such as cIAP1, cIAP2, and XIAP (Fig. 3) [117, 118]. Moreover cFLIP can be upregulated in some cell lines under critical involvement of the NF-κB pathway [119, 120] also resulting in increased resistance to CD95L or TNF.
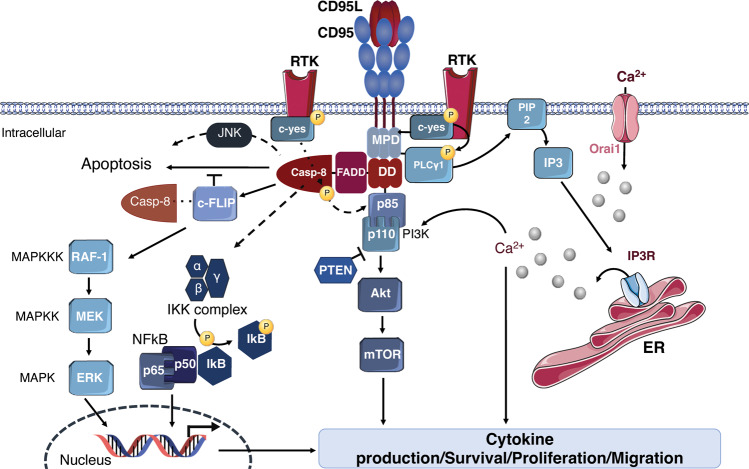
Fig. 3. CD95-dependent non-apoptotic signaling.
Representation of the CD95-mediated unconventional or non-apoptotic pathways. The interaction between CD95 and its ligand CD95L recruits several adaptor proteins leading to the activation of the MAPK, NF-κB and PI3K pathways. The MAPK pathway requires a cascade of phosphorylations to eventually activate ERK, allowing its translocation to the nucleus where it induces the transcription of pro-survival/proliferation/pro-inflammatory genes. The NF-κB heterodimer is kept inactive by IκB, which after IKK-mediated phosphorylation releases NF-κB allowing its translocation to the nucleus where it promotes the transcription of pro-inflammtory/proliferation/migration genes. The PI3K/Akt and PLCy1 pathways are functionally linked in triggering the cell migration. Active PLCy1 participates in the elevation of cytoplasmic calcium levels, which then leads the activation of biochemical pathways that leads to cell proliferation, survival and migration through the phosphorylation and activation of Akt.
MAPK pathway
The MAPK family includes six main groups in humans, among which JNK (Jun N-terminal Kinase), ERK1/2 and the p38 isoform must be mentioned for their involvement in CD95-mediated pro- and anti-apoptotic signaling pathways [121–123]. The induction of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway, which regulates growth, proliferation, differentiation, survival, innate immunity and cellular development is involved in tumorigenesis in multiple tumor types [124]. In the latent state, the inactive MAPKs are cytosolic. The activation of the different MAPKs takes place according to a common general scheme, which provides for a series of sequential phosphorylations catalyzed by different kinases activated in succession. MAPK is phosphorylated by a MAPK kinase (MKK), itself phosphorylated by a MAPK kinase kinase (MKKK), in turn activated by an activator protein.
CD95-mediated stimulation has been suggested early on to induce the JNK pathway through the DAXX adapter protein (Death domain associator protein 6), which after fixation with CD95-DD induces the apoptotic pathway [125, 126]. c-FLIP can block this pathway by inhibiting DAXX [127]. CD95-mediated JNK activation also appears to occur rather slowly, compared to other cell stress stimuli, such as inflammatory cytokines and oxidative stress [128]. Indeed, expression of cFLIP variants or use of different caspase inhibitors in primary human keratinocytes, blocked late death ligand-induced JNK or p38-MAPK activation, suggesting that these responses are secondary to caspase activation [129]. This may be due to the fact that caspase-3 can cleave and thereby activate the MAP3K MEKK1 [128]. Of note, the signal induced by soluble CD95L rather results in a rapid and transient phosphorylation of ERK1/2 [130]. This MAPK protein is widely involved in enhancing growth and proliferation upon CD95 activation [121]. Stimulation of CD95 on primary sensory neurons triggers neurite growth through sustained activation of the extracellular signal-regulated kinase (ERK) pathway and subsequent upregulation of p35, a neurite growth mediator [131]. Of note, in pancreatic apoptosis-resistant tumor cells, CD95L- and TRAIL-induced upregulation of pro-inflammatory genes was found to be partially depend on the ERK signaling pathway via caspase-mediated activation [132]. The same group suggested that the stimulation of the ERKs pathway must probably depend on a caspase-dependent factor operating downstream of the DISC complex. According to another group, CD95L can also induce the autocrine production of EGFR (Epidermal Growth Factor Receptor) ligands and the consequent activation of EGFR followed by ERK1 and ERK2 mitogen-activated protein kinases [133]. In primary fetal astrocytes, blocking ERK phosphorylation with specific inhibitors resulted in a significant reduction of CD95-induced proliferation [134]. In this context, ERK phosphorylation is also caspase-dependent. Noteworthy, cFLIPL can also contribute to ERK activation. Indeed, caspase-8 can cleave cFLIPL into different cleavage products. One of these cleavage products is identified with the name of p43-FLIP [135], which associates with Raf-1 activating the phosphorylation cascade, leading to ERK activation and ultimately to ERK translocation into the nucleus, where it exerts proliferative or pro-inflammatory effects through downstream transcription factor targets (Fig. 3).
PI3K pathway
As mentioned previously, mCD95L can be cleaved by various metalloproteases to produce several soluble forms of the ligand, together referred here as sCD95L [136–140]. Soluble CD95L has been shown to be accumulated in the serum of patients suffering from various diseases [141, 142], whilst the exact cleavage form(s) accumulating in most of these cases remains to be determined. sCD95L was initially believed to be a competitor of its membrane-bound counterpart (mCD95L) in the interaction with CD95 and the consequent induction of the apoptotic signal. It is only in the last decade that it has been reported that not only sCD95L failed in the induction of programmed cell death [143, 144], but that its interaction with CD95 led to the induction of a different type of signal, including engagement of ERK, NF-KB, and PI3K/Akt [145, 146]. Gene-targeted mice selectively lacking either metalloprotease-dependent soluble CD95L (sCD95L) or membrane-bound CD95L (mCD95L) were generated [147]. Mice lacking sCD95L appeared normal and their T cells were able to kill target cells, whereas T cells lacking mCD95L could not kill cells through CD95 activation. Furthermore, mice lacking mCD95L displayed SLE-like symptoms and histiocytic sarcoma. Of note, one group has described that the stimulation of CD95 with sCD95L can induce a calcium-dependent process that leads to the activation of a c-yes/PLCγ1/PI3K/Akt pathway promoting Triple Negative Breast Cancer (TNBC) cell migration [141] (Fig. 3).
mCD95L has also been shown to activate the PI3K/Akt pathway. There appears to be a crosstalk between the two signaling paths PI3K and NF-κB under mCD95L stimulation. Indeed in mutant PIK3CA (PI3K alpha catalytic subunit), but not WT PIK3CA-expressing Hct116 cells, TRAIL, and CD95L stimulated NF-κB activation [148]. It is now clear that caspase-8 not only mediates the cell death signal initiated by CD95L, but also contributes to the induction of apoptosis-independent pathways, such as cell migration and adhesion. Caspase-8 was found to be a substrate of Src kinase c-yes [149]. It has been observed that the stimulation of motility through the EGF, first activates c-yes, and then triggers the phosphorylation of caspase-8 on Tyrosine-380 in the linker region between the two subunits (i.e., p18 and p10) of the procaspase-8 converting it from a pro-apoptotic factor to a cell motility factor. The Y380 phosphorylation prevented downstream activation of the caspase cascade proving a valuable path to explore for sensitization of CD95-resistant tumors to extrinsic apoptotic stimuli [150]. The catalytic domain of caspase-8 is in fact not required for the induction of the migration signal. Once phosphorylated, caspase-8 interacts with the p85 alpha subunit of PI3K (Fig. 3) [151].
In a mouse cell model of glioblastoma (GBM) the c-yes/PI3K-p85 interaction was reported to signal cell invasion via glycogen synthase kinase 3-beta pathway and subsequent expression of matrix metalloproteinases [152]. Blockade of CD95-mediated activity in this cellular model drastically reduced the number of invading cells. In the same context CD95 expression associates with stemness and EMT features and poorer overall survival. CD95-mediated activation of the PI3K p85 also maintained the expression of EMT-related transcripts. The authors therefore suggested that CD95 would be a potential prognostic biomarker in GBM [153].
Systemic autoimmune diseases
To date, more than 80 diseases in which the etiology is certainly, or most likely, autoimmune have been described [154]. Around the 1960s/70s the distinction was made between systemic autoimmune diseases, with general signs and symptoms and the involvement of multiple organs and systems, and organ-specific autoimmune diseases, where the immuno-pathological damage is localized to an organ and the clinical picture closely linked to the dysfunction of the organ itself. The self and non-self recognition functions are carried out through an elaborate identification system that involves T and B lymphocytes. The central selection process eliminates the vast majority of auto-reactive lymphocytes at an immature stage during their development, through Bcl-2-interacting mediators of cell death, such as Bim [155]. However, despite the numerous central tolerance mechanisms, many mature B and T lymphocytes, generated in the central lymphoid organs, then reach the peripheral lymphoid organs and undergo activation, turning into their self-reactive form [156, 157]. The Bim-dependent apoptotic pathway is required both for the killing of self-reactive immature B and T lymphocytes during their development and for the elimination of auto-reactive mature B and T lymphocytes in peripheral lymphoid organs [158]. However, except in the thymus, most of the TCR-related mature T-cell apoptosis is induced by the extrinsic pathway via membrane DR, and in particular one of the most important elements of this regulation is apoptosis activated by the CD95/CD95L system [159]. The importance of CD95 and CD95L in eliminating activated T cells is underlined by the anomalies observed when mutations in the genes coding for CD95 or CD95L occur. The CD95/CD95L system has a dual role in immune regulation [160–163]. It constitutes one of the mechanisms by which cytotoxic lymphocytes kill the target, but is also involved in the process of turning off the response. Activation of the lymphocytes leads to an increase in CD95L expression and the ability to trigger apoptosis. Recently Heikenwälder’s group reported that blocking CD95L could prevent auto-aggression of hepatocytes by CD8pos T cells in the precancerous context of Non-alcoholic steatohepatitis (NASH). The liver cells coming into contact with aberrantly activated CD8pos T cells die by apoptosis due to contact with the CD95L overexpressed in these reactive T cells [164]. The same process could occur and damage other organs as well. This observation, made on a mouse model, could be useful for the design of future immunotherapies without affecting the antigen-specific T-cell immunity.
Peripheral T-cell CD95-induced apoptosis eliminates over-activated and self-reactive T cells via a mechanism called “Activation-Induced Cell Death” (AICD) [165]. T-cell activation is also associated with CD95L expression at the cell surface, thus representing a specific aspect of the immune system. AICD is induced through the interaction between CD95 and CD95L, both expressed on activated T cells surface [166]. Similarly to T cells, it has been reported that not only B cells are capable of expressing CD95L but that the level of CD95L expression correlates with the level of activity of B cells, thus making them capable of killing CD95 expressing cells [167]. Consequently, the abnormal activation of these CD95L-expressing B cells is implicated in the immune modulation of various diseases and thus constitutes a therapeutic target [168, 169]. The constitutive expression of CD95L in some “immunologically privileged” tissues (e.g., the Sertoli cells, the testes, or the anterior chamber of the eye), has suggested that CD95L plays also an important role in reducing the activity of immune cells in these tissues. Several studies exploring mutations in the genes encoding CD95 and CD95L have allowed to better understand the pathogenesis of autoimmune diseases, such as ALPS (Autoimmune LymphoProliferative Syndromes) or SLE (Systemic Lupus Erythematous).
ALPS: Autoimmune lymphoproliferative syndromes
Some FAS gene mutations impair the function of the molecule, leading either to a reduced expression on the membrane, or to the impairment of the ability to transmit the apoptotic signals [170]. The defective shutdown of the immune response resulting from the defective function of CD95 can be the cause of both the progressive accumulation of lymphocytes in the peripheral lymphatic organs and the development of autoimmune reactions [171]. The most common trigger of ALPS is due to autosomal dominant mutations of the FAS gene [172, 173], and, less frequently, of FASLG, the gene encoding the CD95 ligand [174]. Much less common forms of autoimmune lymphoproliferation are due to mutations in another factor in the T-cell apoptosis pathway, caspase-10. Controversial studies have been carried out in this regard as several heterozygous CASP10 variants have been identified along with variants known to be polymorphic. It has recently been observed that said CASP10 mutations are capable of impaired apoptosis [175]. In ALPS patients lacking germline mutations in FAS, some dominant somatic mutations in the DR and notably in the Death Domain were found. These somatic mutations were identified as missense variants likely to change the normal structure and impact the oligomerization and functionality of CD95 [176]. Of note, a large study in a cohort of 100 ALPS patients with CD95 DD mutations reported that the risk of non-Hodgkin and Hodgkin lymphomas, respectively, was 14 and 51 times greater than expected [177]. Collectively, all diseases associated with abnormal lymphocyte apoptosis, lymphoproliferation, and autoimmunity, are named Autoimmune Proliferative Syndromes. The syndromes can be classified according to the mutated gene(s) responsible for the defect and they are usually characterized by lymphadenomegaly and hepatosplenomegaly associated with autoimmune manifestations, mainly of the hematological type, such as hemolytic anemia, thrombocytopenia, and neutropenia, as well as the presence of cell-type-specific autoantibodies [178–180]. Furthermore, the accumulation of a minority population of self-reactive CD3pos TCRαpos CD4neg CD8neg T cells called double negative (DNT) has been reported in the early 1990s as a major feature of ALPS. Despite their similarity to normal differentiated T cells, DNTs are remarkably proliferative, particularly in the paracortical region [181]. Last year Kimberly Gilmour’s group carried out a study on 215 patients with clinical evidence of ALPS, intending to define the most useful and predictable biomarkers for a better ALPS diagnosis. Among the several subgroups of patients, levels of different biomarkers, including DNTs and sCD95L, were observed significantly higher in the ALPS-FAS patients than in the “unknown ALPS” (ALPS-U), cases for which the genetic determinant is not identified. They developed a diagnostic protocol for the potential identification of patients with presymptomatic or mild disease. The combination of such biomarkers could be useful in the process of confirming or excluding the ALPS diagnosis [182]. Today the diagnosis requires performing a cell apoptosis test and molecular type analysis, and the choice of therapy is guided by the severity and nature of the symptoms, but generally it is based on the intake of immunosuppressants such as rituximab. The increased sCD95L serum levels are now part of the new diagnostic criteria procedure for an ALPS [183, 184]; these high levels have indeed been associated with the pathology without their pathophysiological role being elucidated [185]. Curiously, some groups observed a change in sCD95L levels in correlation with aging, and age-related conditions and/or diseases with an increase in molecular signals due to aging oxidative stress [186]. Furthermore, oxidative stress seems to promote CD95L cleavage through activation of MMPs, and more interestingly this MMP activation seems to increase as a function of aging [187].
SLE: Systemic lupus erythematous
In both humans and mice, mutations in the genes coding for CD95 or CD95L are also strongly associated with certain forms of lupus disease. The defects in apoptosis described in autoimmunity and lymphoproliferative syndromes correspond to the human equivalent of the MRL/lpr mouse model (Murphy Roths Large/lymphoproliferation), deeply investigated as a murine SLE-like model [188, 189]. This model was generated following the identification of an autosomal recessive modification on chromosome 19 [190]. The mentioned mutation was found on the gene encoding CD95 protein. Similar to this model, a second model was designed and generated after the discovery of a second autosomal recessive mutation, on chromosome 1, corresponding to the gene coding for CD95L [191]. The latter model took the name of MRL/gld for generalized lymphoproliferative disease. In addition to those two mouse models, a wider selection of mouse models is available to sift genetic and cellular aspects of SLE [192, 193]. Since the etiology of SLE is multifactorial and multigenetic, some of these models, such as those mentioned above, derive from spontaneous genetic factors, while others assume a SLE-like phenotype after exposure to certain chemicals such as intraperitoneal injections of pristane (2, 6, 10, 14 tetramethylpentadecane) [194], or overexpression of cytokines (ie IL-6, IL-12, INF-I) [195–197]. Others, similarly to induced graft-versus-host disease models, develop a lupus-like syndrome following donor cell injection [198]. Despite their numerous limitations, over the years these SLE-like mouse models have been widely used to screen numerous potential therapies, pointing out their importance in the study of this disease and in the therapeutic advancement in this field [199]. Systemic lupus erythematosus is a rare systemic autoimmune disease, more severe in women, especially of childbearing age. Very recently Lars Rönnblom’s group has observed that there is a correlation between the cumulative genetic risk and survival, organ damage, renal dysfunctions, in patients affected by SLE, introducing Genetic Risks Score (GRS) as a potential tool for predicting outcomes in patients with SLE [200]. The term “systemic” means that the disease affects several organs. Genetically speaking, germline heterozygous mutations in the FAS gene have been observed in pediatric cases with ALPS-FAS. These children develop symptoms similar to those of systemic lupus erythematosus disease [201]. According to Neven’s report, FAS mutations were located within the intracellular domain of CD95. On the other hand, germline mutations in the FASLG gene seem to be involved only in a minority of patients with SLE. This does not exclude the possible role of somatic mutations in the FASLG gene in some of the self-reactive clones that contribute to the expression of the disease [202]. High levels of sCD95L have also been detected in the serum of SLE patients, compared to those present in the serum of healthy donors [142]. This observation seems to suggest that high levels of sCD95L may be related to the aggravation of the disease, which constitute a new opening for the study of new therapeutic strategies. Indeed, to date, there are unfortunately no targeted therapies against SLE disease. The diagnosis of this heterogeneous disease is not always simple, as in the early stages the symptoms can simulate other pathologies. For instance, the first “red flags” are given by skin and joint symptoms, both of which can be traced back to multiple pathologies. Less commonly, various infections, as well as pathological conditions such as mixed connective tissue disease (MCTD) or sarcoidosis, can mimic the symptoms of lupus. As a general rule, the first test to be performed is the fluorescence analysis for the detection of antinuclear antibodies (ANA), commonly called autoantibodies. Indeed, 98% of patients with systemic lupus have a positive immunofluorescent ANA test. Several blood and kidney involvement tests are later performed to support the latter. Patients with SLE frequently develop haematopathological and nephropathological conditions, such as leukopenia, thrombocytopenia, hemolytic anemia and active nephritis [203, 204]. The treatment of lupus is standardized and involves corticosteroids, immunosuppressants, and non-steroidal anti-inflammatory drugs in addition to hydroxychloroquine for mild disease [205, 206]. As for new therapeutic options, a large number of drugs, mainly monoclonal antibodies (mAbs), have been evaluated and tested with rather disappointing results. The main objective is to reduce the doses of corticosteroids and immunosuppressants used, as a chronic administration of these drugs causes complications such as infections or secondary osteoporosis [207, 208]. To date, Belimumab (anti-B-cell activating factor) is the only biotherapeutic approved for the treatment of the non-renal form of SLE [209]. The use of Belimumab as an addition to standard therapies seems to improve the quality of life of patients suffering from this disease, but the goal to replace “conventional” drugs remains to be demonstrated. The study conducted on the use of other monoclonal antibodies, for instance Rituximab (anti-CD20) and Anifrolumab (anti-type I interferon receptor), for the treatment of this pathological condition is still ongoing.
Organ-specific autoimmunity
In contrast to systemic autoimmune diseases, organ-specific autoimmunity is characterized by a cell-mediated attack against a specific type of cell in a given target organ, thus causing tissue damage. Some examples of such clinical conditions are insulin-dependent diabetes mellitus, ulcerative colitis (UC), multiple sclerosis (MS), or Sjögren’s syndrome (SS), all conditions to which excessive CD95-mediated apoptosis can contribute [210, 211]. As previously stated, the CD95/CD95L complex plays a central role in controlling immune reactions via AICD. This process is crucial in regulating the autoantigen-dependent primary T-cell response. Therefore, CD95L-mediated AICD dysregulation could be implicated in the acceleration process of organ-specific autoimmune lesions. Furthermore, sCD95L secretion is generally increased in effector cells upon specific activation with organ-specific autoantigen [212]. sCD95L could thus act as an inhibitor of CD95-mediated AICD in these contexts, promoting effector T-cell proliferation and tissue lesions, as demonstrated for antoantigen-reactive CD4 T cells in SS mouse models [212]. Over the years, the numerous studies carried out on the role of the soluble form of CD95L in the context of organ-specific autoimmune diseases, have led to conflicting results. It seems that the role of soluble CD95L varies according to the type of disorder and the mouse model used. In 2019 a group showed on non-obese diabetes mice (NOD) lacking sCD95L and maintaining mCD95L and immune homeostasis that sCD95L does not markedly affect islet inflammation, hence the pathogenesis of autoimmune diabetes, but more interestingly that sCD95L deficiency does not alter immune homeostasis in NOD mice [213].
Currently used cancer therapies
Since the discovery of CD95 [214–217], it has been thought possible to exploit the physiological importance of CD95/CD95L to develop new powerful chemotherapeutic agents. However, it was quite soon discovered that systemic administration of CD95 agonists resulted in severe toxicity [218]. It was observed that these agents induced massive apoptosis of hepatocytes resulting in a form of fulminant hepatitis, lethal to the treated animals [219, 220]. Over the past two decades, controversies over the different implications assumed by the CD95/CD95L system in various diseases such as cancer, autoimmune diseases and inflammatory diseases have made it difficult to identify targeted therapies. Several studies have developed interesting approaches to strengthen the apoptotic function of CD95 and limit the side effects deriving from the non-specificity of the previously developed molecules. Some of these studies will be described in this review. Unfortunately, very few of these approaches have reached clinical trials (Table 1: Breakdown of the patents targeting Death Receptors and/or their ligand).
Table 1.
Table listing the 127 patents published in the last 25 years concerning CD95 or its cognate ligand CD95L or the entire CD95/CD95L interaction system.
| N° | Publication Id | Publication date | Country | Disease | Approach | Title | Inventors | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | WO2003079750 | 2003.10.02 | WO | Autoimmune diseases/inflammatory diseases/cancer | Antibodies | Antagonistic anti-hfas ligand human antibodies and fragments thereof | LANCASTER Joanne Sloan | [350] |
| 2 | US20200102397/WO2017051002 | 2020.04.02/2017.03.30 | WO | Cancer | Antibodies | Anti-cd95l antibody |
GIEFFERS Christian, HILL Oliver, THIEMANN Meinolf et al. |
[221] |
| 3 | WO2015165973 | 2017.03.08 | WO | Cancer | Antibodies | Diagnostic anti-cd95l antibody |
FRICKE, Harald GIEFFERS, Christian SYKORA, Jaromir |
[221] |
| 4 | WO2008080623 | 2016.04.28 | WO | Cancer | Antibodies | Neutralization of cd95 activity blocks invasion of glioblastoma cells in vivo |
MARTIN-VILLALBA Ana, KLEBER Susanne, WIESTLER Benedikt et al. |
[325] |
| 5 | US20150274833/EP2920210/WO2014076292 | 2015.10.01/2015.09.23/2014.05.22 | WO | Cancer | Antibodies | Recombinant bispecific antibody binding to cd20 and cd95 |
HERRMANN Andreas, GROSSE-HOVEST Ludger |
[271, 272, 351] |
| 6 | EP2717911/WO2012168259 | 2014.04.16/2012.12.13 | WO | Cancer | Antibodies | Protein tyrosine phosphatase, non-receptor type 11 (ptpn11) and triple-negative breast cancer |
BENTIRES-ALJ Mohamed, ACETO Nicola, STADLER Michael |
[352] |
| 7 | US20060083738/EP1506237/WO2003097698 | 2006.04.20/2005.02.16/2003.11.27 | WO | Cancer | Antibodies | Treatment of cancer by the use of anti fas antibody |
JOHNSTON Patrick Gerard, LONGLEY Daniel |
[269, 353–355] |
| 8 | WO2010066914 | 2011.10.19 | WO | Others | Antibodies | Remedies for pemphigus containing anti fas-ligand antibodies |
PINCELLI Carlo, MARCONI Alessandra |
[356] |
| 9 | US20030082180/WO2001041803 | 2003.05.01/2001.06.14 | WO | Others | Antibodies | Combination of compounds that inhibit the biological effects of tnf-α and cd95l in a medicament |
KRAMMER Peter, MARTIN-VILLALBA Ana |
[357] |
| 10 | US20110300113/EP2064235/WO2008034608 | 2011.12.08/2009.06.03/2008.03.27 | WO | Others | Antibodies/cells | The death receptor cd95 controls neurogenesis of adult neural stem cells in vivo and in vitro |
MARTIN-VILLALBA Ana, CORSINI Nina, LETELLIER Elisabeth et al. |
[358] |
| 11 | WO2010006772 | 2011.08.04 | WO | Inflammatory diseases | Antibodies/fusion proteins | Use of cd95 inhibitors for the treatment of inflammatory disorders |
MARTIN-VILLALBA Ana, LETELLIER Elisabeth, SANCHO-MARTINEZ Ignacio |
[359] |
| 12 | US20170166648/EP3150224/WO2014013036 | 2017.06.15/2017.04.05/2014.01.23 | WO | Others | Antibodies/fusion proteins | Inhibitors of the cd95 signaling pathway for treatment of mds |
FRICKE Harald, FONTENAY Michaela, KUNZ Claudia |
[348, 349] |
| 13 | US20060234968/WO2004071528 | 2006.10.19/2004.08.26 | WO | Others | Antibodies/fusion proteins | Inhibition of the cd95 ligand/receptor system for the treatment of neurological disorders and injuries |
MARTIN-VILLALBA Ana, KRAMMER Peter, DEMJEN Deana |
[360] |
| 14 | US20030170244 | 2003.09.11 | US | Cancer/others | Antibodies/fusion proteins | Inhibition of fas signaling |
PLUENNEKE John D, CONNOR Timothy |
[361] |
| 15 | US6846637/WO1999065935 | 2005.01.25/1999.12.23 | WO | Cancer/autoimmune diseases/others | Antibodies/polypeptides | Fas peptides and antibodies for modulating apoptosis | CHIODI Francesca | [362] |
| 16 | WO2016170027 | 2016.10.27 | WO | Inflammatory diseases | Antibodies/polypeptides | Methods and pharmaceutical compositions for the treatment of th17 mediated diseases |
LEGEMBRE Patrick, BLANCO Patrick, FLYNN Robin |
[101] |
| 17 | US20120294856/EP2502069/WO2011058175 | 2012.11.22/2012.09.26/2011.05.19 | WO | Cancer | Antobodies/enzymes/RNA interfering molecules | Compounds inhibiting cd95 signaling for the treatment of pancreatic cancer |
MARTIN-VILALBA Ana, HERHAUS Peter, SANCHO-MARTINEZ Ignacio et al. |
[363] |
| 18 | US20200407728/WO2016069282 | 2020.12.31/2016.05.06 | WO | Autoimmune diseases | Cells | Altering gene expression in modified t cells and uses thereof |
ZHAO Yangbing, REN Jiangtao, LIU Xiaojun, JUNE Carl H. |
[364] |
| 19 | US20150104428/EP2833896/WO2013149211 | 2015.04.16/2015.02.11/2013.10.03 | WO | Autoimmune diseases | Cells | Compositions and treatment methods for mesenchymal stem cell-induced immunoregulation |
SHI Songtao, AKIYAMA Kentaro, CHEN Chider |
[330, 331] |
| 20 | WO2015038665 | 2015.03.19 | WO | Autoimmune diseases/inflammatory diseases | Cells | A composition of stem cells having highly expressed fas ligand |
SHI Songtao, LIU Shiyu, CHEN Fa-ming |
[365] |
| 21 | US20200121719/EP3565888/WO2018129332 | 2020.04.23/2019.11.13/2018.07.12 | WO | Cancer | Cells | Expansion of tumor-infiltrating lymphocytes (tils) with tumor necrosis factor receptor superfamily (tnfrsf) agonists and therapeutic combinations of tils and tnfrsf agonists |
LOTZE Michael, T, RITTHIPICHAI Krit |
[277, 366, 367] |
| 22 | EP3569700/WO2011052545 | 2019.11.20/2011.05.05 | WO | Cancer | Cells | Method for producing antigen-specific b-cell population | KITAMURA Daisuke, NOJIMA Takuya | [368] |
| 23 | US20180008670 | 2018.01.11 | US | Cancer | Cells | Chimeric antigen receptor targeting of tumor endothelium |
WAGNER Samuel C., ICHIM Thomas E, MINEV Boris |
[280] |
| 24 | WO2015161276 | 2015.10.22 | WO | Cancer | Cells | Crispr-cas-related methods, compositions and components for cancer immunotherapy | WELSTEAD G. Grant, FRIEDLAND Ari E, MAEDER Morgan L, BUMCROT David A | [369] |
| 25 | WO2014039044 | 2014.13.03 | US | Cancer | Cells | Methods of producing t memory stem-cell populations |
GATTINONI Luca, LUGLI Enrico, ROEDERER Mario, RESTIFO Nicholas P |
[281, 370, 371] |
| 26 | US20040131599/WO2002072798 | 2004.07.08/2002.09.19 | WO | Cancer | Cells | Fas ligand-expressing hematopoietic cells for transplantation |
CIVIN Curt, I, DRACHMAN Daniel, WHARTENBY Katherine, PARDOLL Drew M |
[372] |
| 27 | US20090191167/EP2046351/WO2008014470 | 2009.07.30/2009.04.15/2008.01.31 | WO | Cancer/autoimmune diseases/others | Cells | Adult sertoli cells and uses thereof | WHITE David J | [373] |
| 28 | WO2018227286 | 2018.12.20 | WO | Inflammatory diseases/others | Cells | Allograft tolerance without the need for systemic immune suppression |
NAGY Andras, HARDING Jeffrey, NAGY Kristina |
[374] |
| 29 | WO2018130679 | 2019.11.20 | WO | Cancer | Chemicals | Methods and pharmaceutical compositions for reducing cd95- mediated cell motility |
LEGEMBRE Patrick, VACHER Pierre, POISSONNIER Amanda, BLANCO Patrick |
[101] |
| 30 | US20190084987 | 2019.03.21 | US | Cancer | Chemicals | Small molecule histone methyltransferase suv39h1 inhibitor and uses thereof |
LU Chunwan, LEBEDYEVA Iryna, LIU Kebin |
[301] |
| 31 | US20150343024/EP2931375/WO2014090224 | 2015.12.03/2015.10.21/2014.06.19 | WO | Cancer | Chemicals | Use of active substance combinations for inducing tumor senescence |
RÖCKEN Martin, WIEDER Thomas, HAHN Matthias et al. |
[375] |
| 32 | WO2008036244 | 2008.03.27 | WO | Cancer | Chemicals | Use of cyclosporin a to sensitize resistant cancer cells to death receptor ligands |
REED John C, THOMAS Michael P |
[376] |
| 33 | WO2019206834 | 2019.10.31 | WO | Cancer/autoimmune dieases | Chemicals | Compounds and pharmaceutical compositions for reducing cd95-mediated cell motility |
VACHER Pierre, LEGEMBRE Patrick, JEAN Mickael et al. |
[142] |
| 34 | WO2002047728 | 2002.06.20 | WO | Others | Chemicals | Treatment of posterior capsule opacification | ALLAN Bruce Duncan Samuel | [377] |
| 35 | WO2019141862 | 2019.07.25 | WO | Inflammatory diseases | Chemicals/antobodies/fusion proteins/nucleotide complexes | Combination therapeutics |
WALCZAK Henning, TARABORRELLI Lucia, PELTZER Nieves |
[321] |
| 36 | US20090142369 | 2009.06.04 | US | Others | Cosmetic composition | Method for preventing skin-cellular injury by using green algae extract and cosmetic composition containing green algae extract |
SHIH Meng-Han, SHIH Mei-Fen |
[378] |
| 37 | US20080118466 | 2008.05.22 | US | Autoimmune diseases | Drug delivery system | Treatment of rheumatoid arthritis with soluble fas-ligand cross-linkers |
HUREZ Vincent Jacques, MICHELSON Seth G, SHODA Lisl Katharine et al. |
[379, 380] |
| 38 | EP0930890/WO1998017305 | 1999.07.28/1998.04.30 | WO | Autoimmune diseases | Drug delivery system | Use of fasl or fasl transfected cd4?+ /fasl?- /th1-cell lines for the treatment of th1/th2 diseases |
HAHNE Michael, TSCHOPP Juerg, DA CONCEICAO-Silva Fatima, SCHROETER Michael |
[381] |
| 39 | US20200046780/EP3592392 | 2020.02.13/2020.01.15 | US/EP | Autoimmune diseases/others | Drug delivery system | Fasl-engineered biomaterials with immunomodulatory function |
SHIRWAN Haval, GARCIA Andres J, YOLCU Esma S et al. |
[136, 382] |
| 40 | US20050214311/EP1478390/WO2003070271 | 2005.09.29/2004.11.24/2003.08.28 | WO | Cancer | Drug delivery system | Novel complexes for inducing an immune response |
SCREATON Gavin R, SIMON Katharina A, GALLIMORE Awen M |
[383] |
| 41 | US20200108117/WO2019246130 | 2020.04.09/2019.12.26 | US | Cancer/others | Drug delivery system | Drug delivery systems comprising an intraocular pressure lowering agent, a neurotrophic agent, a c-type natriuretic peptide, a natriuretic peptide receptor-b, an apoptosis signaling fragment inhibitor or a fas-ligand inhibitor for treating glaucoma or ocular hypertension | SCHIFFMAN Rhett M, SCHEIBLER Lukas | [384] |
| 42 | US20200246432/WO2019040372 | 2020.08.06/2019.02.28 | WO | Others | Drug delivery system | Nitric oxide- and fas ligand- eluting compositions and devices and methods of treatment using same |
KURAL M Hamdi, GUI Liqiong, NIKLASON L Elizabeth, SALTZMAN William Mark |
[385] |
| 43 | WO2019246141 | 2019.12.26 | WO | Others | Drug delivery system | Drug delivery systems comprising a neurotrophic agent, an apoptosis signaling fragment inhibitor (fas) or fas-ligand (fasl) inhibitor, a tumor necrosis factor-α (tnf-α) or tnf receptor inhibitor, a mitochondrial peptide, an oligonucleotide, a chemokine inhibitor, or a cysteine-aspartic protease | SCHIFFMAN Rhett M, SCHEIBLER Lukas | [384] |
| 44 | WO2019246130 | 2019.12.26 | WO | Others | Drug delivery system | Sustained-release drug delivery systems comprising an intraocular pressure lowering agent, a cnp compound, an npr-b compound, a tie-2 agonist, or neurotrophic agent for use for treating glaucoma or ocular hypertension | SCHIFFMAN Rhett M, SCHEIBLER Lukas | [384] |
| 45 | US20040096450/WO2000040263 | 2004.05.20/2000.07.13 | WO | Others | Drug delivery system | Methods and compositions for treating diseases associated with increased fas-ligand titers |
FRENCH Lars E, VIARD Isabelle, TSCHOPP Jurg |
[386] |
| 46 | US20190330305/WO2014121085 | 2019.10.31/2014.08.14 | WO | Autoimmune diseases/inflammatory diseases/cancer | Fusion proteins | Pd-l1 and pd-l2-based fusion proteins and uses thereof | TYKOCINSKI Mark L | [387] |
| 47 | EP1481687 | 2004.12.01 | EP | Autoimmune diseases/inflammatory diseases/cancer | Fusion proteins | Use of multimeric ligands of the tnf family with reduced toxicity for treating cell proliferative diseases | ROSAT Jean-Pierre | [294] |
| 48 | US20070269449/WO2004085478 | 2007.11.22/2004.10.07 | WO | Autoimmune diseases/inflammatory diseases/others | Fusion proteins | Cd95-fc fusion proteins | WALCZAK Henning | [388] |
| 49 | EP1214411/WO2001018202 | 2002.06.19/2001.03.15 | WO | Autoimmune diseases/inflammatory diseases/others | Fusion proteins | Flint analog compounds and formulations thereof |
ATKINSON Paul Robert, TIAN Yu, WITCHER Derrick Ryan |
[389–391] |
| 50 | WO2001090382 | 2001.11.29 | WO | Autoimmune diseases/inflammatory diseases/others | Fusion proteins | Fas ligand-fused proteins | TOUMA Jyunko | [392] |
| 51 | US20180318394/EP3313429/WO2016205714 | 2018.11.08/2018.05.02/2016.12.22 | WO | Autoimmune diseases/others | Fusion proteins | Immunomodulation for the long-term prevention and treatment of autoimmune diseases and foreign tissue rejection | SHIRWAN Haval | [382, 393, 394] |
| 52 | US20110081369/EP1250055 | 2011.04.07/2002.10.23 | US/EP | Autoimmune diseases/others | Fusion proteins | Methods of immune modulation with death receptor-induced apoptosis | SHIRWAN Haval | [382, 393, 394] |
| 53 | US20110003385/EP0804561 | 2011.01.06/1997.11.05 | US/EP | Autoimmune diseases/others | Fusion proteins | Regulated transcription of targeted genes and other biological events | CRABTREE Gerald R, SCHREIBER Stuart L, SPENCER David M | [395] |
| 54 | US20090239240/EP2039768 | 2009.09.24/2009.03.25 | US | Autoimmune diseases/others | Fusion proteins | Mutant forms of fas ligand and uses thereof | KETING Chu | [266] |
| 55 | US20180148512/WO2014121093 | 2018.05.31/2014.08.07 | WO | Cancer | Fusion proteins | Fusion proteins that facilitate cancer cell destruction | TYKOCINSKI Mark L | [289] |
| 56 | WO2015197874 | 2017.05.03 | WO | Cancer | Fusion proteins | Combination of cd95/cd95l inhibition and cancer immunotherapy |
KUNZ Claudia, FRICKE Harald, HÖGER Thomas, GAMER Juergen |
[266, 396–398] |
| 57 | US20150297745/EP2897642/WO2014045022 | 2015.10.22/2015.07.29/2014.03.27 | WO | Cancer | Fusion proteins | Agents and methods |
COBBOLD Mark, MILLAR David |
[399] |
| 58 | WO2012170072 | 2014.04.17 | WO | Cancer | Fusion proteins | Engineered antibody-tnfsf member ligand fusion molecules |
GREWAL Iqbal, KHARE Sanjay D, GRESSER Michael, SYED Rashid |
|
| 59 | US20120177575/EP2456468/WO2011010156 | 2012.07.12/2012.05.30/2011.01.27 | WO | Cancer | Fusion proteins | Fas (apo-1,cd95) targeted platforms for intracellular drug delivery |
ATEH Davidson D, MARTIN Joanne E |
[400] |
| 60 | US20110008842 | 2011.01.13 | US | Cancer | Fusion proteins | Chimeric nucleic acids encoding polypeptides comprising cd70 and fas-ligand domains |
PRUSSAK Charles E, KIPPS Thomas J, CANTWELL Mark J |
[401] |
| 61 | EP3406630/US20180186856/WO2014013037 | 2018.11.28/2018.02.22/2014.01.23 | WO | Cancer/autoimmune diseases/inflammatory diseases/others | Fusion proteins | Nucleic acids encoding artificial signal peptides and methods of production thereof |
HILL Oliver, GIEFFERS Christian, THIEMANN Meinolf |
[324, 325, 346, 348, 402] |
| 62 | WO2014013039 | 2014.01.23 | WO | Cancer/autoimmune diseases/inflammatory diseases/others | Fusion proteins | Composition comprising a mixture of cd95-fc isoforms |
HILL Oliver, GIEFFERS Christian, THIEMANN Meinolf |
[324, 325, 402] |
| 63 | WO2013060864 | 2014.09.03 | WO | Cancer/autoimmune diseases/others | Fusion proteins | Chimeric molecule involving oligomerized fasl extracellular domain |
TAUPIN Jean-Luc, DABURON Sophie, MOREAU Jean-François, CAPONE Myriam |
[292, 293] |
| 64 | WO2008025516 | 2011.02.03 | WO | Cancer/inflammatory diseases/others | Fusion proteins | Cd95l or trail fusion proteins |
HILL Oliver, GIEFFERS Christian, THIEMANN Meinolf |
[324, 325, 402] |
| 65 | US20190016782/WO2014106839 | 2019.01.17/2014.07.10 | WO | Cancer/others | Fusion proteins | Stable form of signal converting protein fusion proteins, and methods of use and preparation thereof |
DRANITZKI ELHALEL Michal, SHANI Noam |
[266] |
| 66 | EP2042509/US20070154905 /WO1997003998 | 2009.04.01/2007.07.05/1997.02.06 | WO | Cancer/others | Fusion proteins | Modulators of the function of fas receptors and other proteins |
WALLACH David, BOLDIN Mark, GONCHAROV Tanya, GOLSTEV Yury V |
[403] |
| 67 | WO2007022273 | 2007.02.22 | WO | Cancer/others | Fusion proteins | Vegf-activated fas ligands | QUINN Timothy P | [404, 405] |
| 68 | US20040147447/WO1999066039 | 2004.07.29/1999.12.23 | WO | Cancer/others | Fusion proteins | Tnfr-like protein with death domain |
LU Jian J, GOMES Bruce C, FIELES William E |
|
| 69 | US20110171212 | 2011.07.14 | US | Inflammatory diseases | Fusion proteins | Methods and compositions for preventing radiation-induced pneumonitis |
BELKA Claus HERBST Jörg |
[406] |
| 70 | US20040176279/WO2002060949 | 2002.08.08 | WO | Inflammatory diseases/others | Fusion proteins | Glycoforms a fas-ligand inhibitory protein analog |
JENKINS Nigel, WITCHER Derrick R WROBLEWSKI Victor J |
[407] |
| 71 | US20160340409/EP2621514/WO2012042480 | 2016.11.24/2013.08.07/2012.04.05 | WO | Others | Fusion proteins | Compositions and methods for treatment of hematological malignancies | DRANITZKI ELHALEL Michal | [266] |
| 72 | US20040018170 | 2004.01.29 | US | Others | Fusion proteins | Fas ligand-avidin/streptavidin fusion proteins | SHIRWAN Haval | [382, 393, 394] |
| 73 | EP1097226/WO2000003023 | 2001.05.09/2000.01.20 | WO | Cancer/autoimmune diseases/others | Fusion proteins | Usurpin, a mammalian ded-caspase homologue that precludes caspase-8 recruitment and activation by the cd95 (fas, apo-1) receptor complex |
NICHOLSON Donald, W, RASPER Dita M, XANTHOUDAKIS Steve, ROY Sophie |
[408] |
| 74 | EP3337509/US20170051352 | 2018.06.27/2017.02.23 | US/EP | Autoimmune diseases | Method | Methods of treating autoimmune conditions in patients with genetic variations in dcr3 or in a dcr3 network gene |
HAKONARSON Hakon, KAO Charlly, CARDINALE Christopher et al. |
[409] |
| 75 | US20160194714 | 2016.07.07 | US | Autoimmune diseases | Method | Biomarkers for predicting relapse in multiple sclerosis |
RUS Horea TEGLA Cosmin |
[306–309] |
| 76 | US20160208332 | 2016.04.07 | US | Autoimmune diseases | Method | Diagnosis and prognosis of multiple sclerosis |
RUS Horea CUDRICI Cornelia TEGLA Cosmin |
[306–309] |
| 77 | US20100285600/EP1891233/WO2006116602 | 2010.11.11/2008.02.27/2006.11.02 | WO | Autoimmune diseases | Method | Markers associated with the therapeutic efficacy of glatiramer acetate |
LANCET Doron BECKMANN Jacques AVIDAN Nili et al. |
[410] |
| 78 | US20090074870/WO2002002751 | 2009.03.19/2002.01.10 | WO | Autoimmune diseases/others | Method | Alteration of cell membrane with fasl | SHIRWAN Haval | [411] |
| 79 | WO2015107105 | 2016.11.23 | WO | Cancer | Method | Method of predicting the responsiveness of a cancer disease to treatment on the basis of dna methylation | FRICKE Harald | [297–299] |
| 80 | WO2015104284 | 2016.11.16 | WO | Cancer | Method | Methods and pharmaceutical compositions for preventing or reducing metastatic dissemination |
LEGEMBRE Patrick, SEGUI Bruno, LEVADE Thierry, MICHEAU Olivier |
[296, 412] |
| 81 | WO2014118317 | 2015.12.31 | WO | Cancer | Method | Methods for predicting and preventing metastasis in triple-negative breast cancers |
LEGEMBRE Patrick, MALLETER Marine, TAUZIN Sébastien et al. |
[141] |
| 82 | US20150098924/WO2006037762 | 2015.04.09/2006.04.13 | WO | Cancer | Method | Method for ex-vivo purging in autologous transplantation |
DUPUIS Marc, GREANEY Peter, DUCHOSAL Michel |
[413] |
| 83 | EP1668360/US20050069963 | 2006.06.14/2005.03.31 | US/EP | Cancer | Method | Multifactorial assay for cancer detection |
LOKSHIN Anna E. GORELIK Elieser |
[414] |
| 84 | US20050158807/WO2003056340 | 2005.07.21/2003.07.10 | WO | Cancer | Method | Fadd proteins, phosphorylated p38-mapk and fasl as tumor markers |
CHIOCCHIA Gilles, TOURNEUR Lea, FEUNTEUN Jean et al. |
[415] |
| 85 | WO2005053739 | 2005.06.16 | WO | Cancer | Method | Combination therapy |
JOHNSTON Patrick G, LONGLEY Daniel |
[416] |
| 86 | EP1127075/WO2000027883 | 2001.08.29/2000.05.18 | WO | Cancer | Method | A method of treating tumors using fas-induced apoptosis |
DONG Jian-Yun NORRIS James S |
[417] |
| 87 | WO2001048238 | 2001.07.05 | WO | Cancer | Method | Chemotherapeutant screening method |
KRAMMER Peter, EICHHORST Sören, LI-WEBER Min, MÜLLER-SCHILLING Martina |
[418] |
| 88 | WO1999003998 | 1999.01.28 | WO | Cancer | Method | Methods and compositions for tumor reduction | NABEL Gary J | [419] |
| 89 | US6153385/WO1997020067 | 2000.11.28/1997.06.05 | WO | Cancer/autoimmune diseases/others | Method | Process for detecting the expression of cd95 ligand in cells |
DEBATIN Klaus-Michael HERR Ingrid |
[420] |
| 90 | EP0876503/WO1997020064 | 1998.11.11/1997.06.05 | WO | Cancer/autoimmune diseases/others | Method | Process for assessing the activity of drugs |
DEBATIN Klaus-Michael, FRIESEN Claudia, KRAMMER Peter, HERR Ingrid |
[420] |
| 91 | EP0689600/WO1994020625 | 1996.01.03/1994.09.15 | WO | Cancer/others | Method | Process to induce the death of tumor cells | WONG Grace H W | [421] |
| 92 | WO1999003999 | 1999.01.28 | WO | Inflammatory diseases | Method | Methods and compositions for inhibiting the pro-inflammatory response |
NABEL Gary J, CHEN Jian-Jun |
[422] |
| 93 | US20020127233 | 2002.09.12 | US | Inflammatory diseases/cancer/others | Method | Method for inhibiting inflammation in immune privileged sites using fas-ligand fragments |
ZHU Bing, CYNADER Max S, PATY Donald W, LUO Liqing |
[423] |
| 94 | US20180369380 | 2018.12.27 | US | Others | Method | Methods and compositions for treating conditions of the eye |
GRAGOUDAS Evangelos S, POULAKI Vasiliki, MILLER Joan W |
[424] |
| 95 | US20140045198/EP2678688 /WO2012113760 | 2014.02.13/2014.01.01/2012.08.30 | WO | Others | Method | Method of predicting the evolution of a patient suffering of a neurovascular disease |
MONTANER VILLALONGA Joan, ROSELL NOVEL Anna, NAVARRO SOBRINO Miriam |
[357, 425] |
| 96 | US20110294690/EP2338058/WO2010031821 | 2011.12.01/2011.06.29/2010.03.25 | WO | Others | Method | Differential diagnostic biomarkers of stroke mimicking conditions and methods of use thereof | MONTANER VILALLONGA Joan | [357, 425] |
| 97 | US20060241150/WO2005000405 | 2006.10.26/2005.01.06 | WO | Others | Method | P38 kinase inhibitor compositions and methods of use |
WEINER David B, MUTHUMANI Karuppiah |
[426] |
| 98 | WO2006077232 | 2006.07.27 | WO | Others | Method | Multimeric soluble fas-ligand for eliminating alloreactive t lymphocyte in allogenic harmatopoietic stem-cell transplantation transplantation |
DUPUIS Marc, DEMOTZ Stéphane, GREANEY Peter et al. |
[413, 427] |
| 99 | US20050129684 | 2005.06.16 | US | Others | Method | Methods for preserving the viability of photoreceptor cells by anti-fas-ligand/anti-fas-receptor antibodies |
ZACKS David, MILLER Joan W |
[428] |
| 100 | US20030224403 | 2003.12.04 | US | Others | Method | Lethal toxin cytopathogenicity and novel approaches to anthrax treatment |
POPOV Serguei G, CARRON Edith G, CARDWELL Jennifer |
[429] |
| 101 | US6524821/WO2000007618 | 2003.02.25/2000.02.17 | WO | Others | Method | Anti-apoptotic compositions comprising the r1 subunit of herpes simplex virus ribonucleotide reductase or its n-terminal portion; and uses thereof |
LANGELIER Yves, MASSIE Bernard |
[430] |
| 102 | US6485929/WO1999036091 | 2002.11.26/2000.07.22 | WO | Others | Method | Method for inhibiting cd95-independent apoptosis in aids |
KRAMME, Peter H, BERNDT Christina |
[431] |
| 103 | WO2015189236 | 2015.12.17 | WO | Cancer | Method | Methods and pharmaceutical compositions for reducing cd95-mediated cell motility |
LEGEMBRE Patrick, COUNILLON Laurent, LAGADIC-GOSSMANN Dominique |
[295] |
| 104 | US2003011865/WO2000059538 | 2002.06.06/2000.10.12 | WO | Autoimmune diseases/others | Nucleotide complexe | Antigen-specific induction of peripheral immune tolerance |
AUGUST Thomas J. LEONG Kam W. GEORGANTAS Robert |
[432] |
| 105 | WO2001051503 | 2001.07.19 | WO | Cancer | Nucleotide complexe | Polynucleotides for inhibiting metastasis and tumor cell growth | BARBERA-GUILLEM Emilio | [433] |
| 106 | US20020042064/EP1121438/WO2000023583 | 2002.04.11/2001.08.08/2000.04.27 | WO | Cancer/autoimmune diseases/others | Nucleotide complexe | P53 binding areas |
KRAMMER Peter, MÜLLER-SCHILLING Martina, OREN Moshe |
[251] |
| 107 | US20040033979/EP1176965 | 2004.02.19/2002.02.06 | US/EP | Cancer/autoimmune diseases/inflammatory diseases | Nucleotide complexe | Antisense modulation of fas mediated signaling |
DEAN Nicholas M, MARCUSSON Eric G, WYATT Jacqueline, ZHANG Hong |
[434] |
| 108 | US20030119776/EP1313853 | 2003.06.26/2003.05.28 | US/EP | Cancer/autoimmune diseases/others | Nucleotide complexe | Modulation of fas and fasl expression |
PHILLIPS Nigel C, FILION Mario C |
[435] |
| 109 | US20070190607/WO2001058953 | 2007.08.16/2001.08.16 | WO | Autoimmune diseases | Polypeptides | Inhibitors of pre-ligand assembly doman and function of the tumor necrosis factor receptor family |
LENARDO Michael J, CHAN Francis Ka-Ming, SIEGEL Richard M |
[36] |
| 110 | US7097972/WO1996025501 | 2006.08.29/1996.08.22 | WO | Autoimmune diseases/inflammatory diseases/cancer | Polypeptides | Method and composition for regulating apoptosis | DIXIT,Vishva M | [436] |
| 111 | US20120245081 | 2012.09.27 | US | Autoimmune diseases/inflammatory diseases/others | Polypeptides | Fas peptide mimetics and uses thereof |
GREENE Mark I, MURALI Ramachandran, HASEGAWA Akihiro |
[437] |
| 112 | US20070184522/EP1737483/WO2005117940 | 2007.08.09/2007.01.03/2005.12.15 | WO | Autoimmune diseases/others | Polypeptides | Cell death modulation via antagonists of fasl and fas activation |
ZARNEGAR Abdolreza, DEFRANCES Marie C, ZOU Chun-Bin |
[438] |
| 113 | US6451759/WO1999036079 | 2002.09.17/1999.07.22 | WO | Autoimmune diseases/others | Polypeptides | Noncleavable fas ligand |
KANG, Sang-Mo BRAAT, Dries BAEKKESKOV, Steinunn STOCK, Peter, G. |
[439] |
| 114 | WO2020132465 | 2020.06.25 | WO | Cancer | Polypeptides | Methods and compositions related to therapeutic peptides for cancer therapy |
BECKER Lev, CUI Chang |
[286] |
| 115 | EP2102234/WO2008067305 | 2009.09.23/2008.05.06 | EP | Cancer | Polypeptides | Polypeptides comprising intracytoplasmic death domain and nkg2d ligand domain |
WAGNER Thomas E, WEI,Yanzhang |
[440] |
| 116 | US20190085050/WO2015158810 | 2019.03.21/2015.10.22 | WO | Cancer/autoimmune diseases/inflammatory diseases | Polypeptides | Polypeptides and uses thereof for reducing cd95-mediated cell motility |
LEGEMBRE Patrick, VACHER Pierre, SANSEAU Doriane et al. |
[146] |
| 117 | EP1225908/WO2001028582 | 2002.07.31/2001.04.26 | WO | Cancer/inflammatory diseases/others | Polypeptides | Therapeutic applications of flint polypeptides |
BUMOL Thomas F COHEN Fredric J |
|
| 118 | US20100041596/WO2007002633 | 2010.02.18/2007.01.04 | WO | Inflammatory diseases | Polypeptides | Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain (plad) of tumor necrosis factor receptors |
LENARDO Michael, DENG Guo-Min, CHAN Francis Ka-Ming, ZHENG Lixen |
[441] |
| 119 | US20100041596/WO2009027350 | 2017.08.31/2009.03.05 | WO | Inflammatory diseases/others | Polypeptides | Use of sco-spondin peptides for inhibiting or preventing neuronal apoptosis mediated by cell death receptor ligands |
MEINIE, Annie, LALLOUE Fabrice, JAUBERTEAU Marie-Odile |
[442] |
| 120 | US20130288979/EP2982685 /WO2012066103 | 2017.11.14/2016.02.10/2012.05.24 | WO | Others | Polypeptides | Inhibitors of apoptosis and uses thereof |
BARRERE Stéphanie, NARGEOT Joël, LEBLEU Bernard et al. |
[443] |
| 121 | US20010018416 | 2001.08.30 | US | Others | Polypeptides | Compositions and methods for treating hepatitis-c |
SLESAREV Vladimir I, DIMITROV Todor |
[444] |
| 122 | US20060089491 | 2004.06.07 | US | Cancer | Polypeptides/nucleotide complexes | Fas-ligand derived polypeptides |
NAGATA Shigekazu, SUDA Takashi, TAKAHASHI Tomohiro, NAKAMURA Norio |
[445] |
| 123 | US20090169599 | 2009.07.02 | US | Others | Reprogrammed virus | Scientifically modulated and reprogrammed treatment (smart) fas/fasl virus technology intended to neutralize t-helper cells infected with the human immunodeficiency virus |
SCHEIBER Lane Bernard SCHEIBER II Lane Bernard |
|
| 124 | EP2355833/US20100324116 | 2011.08.17/2010.12.23 | US/EP | Cancer | RNA interfering molecules | Fas/fasl or other death receptor targeted methods and compositions for killing tumor cells |
KRUSE Carol, TRITZ Richard |
[283] |
| 125 | US20050119212 | 2004.06.18 | US | Autoimmune diseases/others | RNA interfering molecules | Rna interference mediated inhibition of fas and fasl gene expression using short interfering nucleic acid (sina) |
HAEBERLI Peter, MCSWIGGEN James |
[446] |
| 126 | US20070004666 | 2007.01.04 | US | Cancer/autoimmune diseases/others | Transcription factors | Methods for modulating apoptotic cell death |
LASHAM Annette, WATSON James D |
[447, 448] |
| 127 | WO1998008965 | 1998.03.05 | WO | Autoimmune diseases/others | Transcription factors/nucleotide complexes | Cd95 regulatory gene sequences and transcription factors |
WATSON James, D, RUDERT Fritz |
[447, 448] |
The source of information used is the Patentscope of the WIPO IP Portal and the search was carried out by selecting only the patents published in the United States Patent Office and European Patent Office. Furthermore, the selection was made using keywords and selecting only the patents with these keywords in the title of the publication or the corresponding abstract on the front page. The keywords used are CD95, CD95L, CD95 ligand, Fas, FasL, Fas ligand.
It is now well established that the apoptotic signal is often defective in cancer and that the CD95/CD95L interaction is involved in the tumor cells’ escape from the immune surveillance system. For instance, some tumor cell types [221], i.e., some cancer cells, effector T cells (CD8pos), regulatory T cells (CD4pos, CD25pos), tumor endothelial cells, Myeloid-derived suppressor cells (MDSC), Monocyte-derived human macrophages (MDM), Cancer-associated fibroblasts (CAF), Cancer stem cells (CSC), are able to express CD95L at the membrane, thus conferring the tumor environment a “counterattack” mechanism involved in the elimination of tumor-infiltrating lymphocytes and prevention of successful immunotherapy [222–228]. Interestingly, it was observed that the vascular endothelial cells of some solid tumors also express the membrane-bound form of CD95L through a mechanism involving tumor-derived vascular endothelial growth factor A (VEGF-A), interleukin 10 (IL-10) and prostaglandin E2 (PGE2) [229]. CD95L expression becomes here a defense barrier against CD8pos T cells, preventing their extravasation and their access to the tumor nest [230]. Furthermore, it has been observed that different types of cancer cells release vesicles called Tumor-Derived Exosome (TEX) into the microenvironment, which act as messengers between cells. TEX can carry several immunosuppressive molecules, including membrane-bound CD95L. This represents a further defense mechanism by the tumor cells against the CD8pos T cells that manage to infiltrate the tumor nest [231]. TEX can inhibit the proliferation of CD8pos T cells by apoptotic induction, thus playing a major role in immune evasion [232, 233]. In this context, engineered exosomes appears interesting to design potential immunotherapies such as cancer vaccines [234]. Moreover, inhibition of CD95L could prevent cancer resistance to radiotherapeutic or immunotherapeutic treatments, thus representing another path to follow in cancer immunotherapy. Today it is possible to predict, assess and monitor whether a subject with cancer is sensitive to treatment with immunotherapeutics. The Gustave Roussy institute has published a method and kits to determine if in a sample of a said subject one or more biomarkers, including CD95pos CD4pos T cells, CD95pos CD8pos T cells is present/absent together with its expression level (WO2017140826). In 2019, the soluble metalloprotease-cleaved CD95L, associated with a large number of immune infiltrate cells, has been identified as a possible biomarker for tumor immune infiltration (CD3pos and CD8pos, and also CD4 and FoxP3 T cells) in advanced HGSOC (High-Grade Serous Ovarian Cancer) [235]. These biomarkers can facilitate the identification of cancer patients prone to respond or resist to proposed immunotherapy and therefore to select an appropriate and personalized chemotherapy treatment. In the last 20 years, the strategies adopted in cancer immunotherapy can be classified into the two large families of active and passive immunotherapy.
Active approaches
Active immunotherapy is based on the principle that the drug stimulates the patient’s immune response against the tumor, thus acting indirectly. On the contrary, in the case of passive immunotherapy, the drug is directly capable of destroying the tumor cell. Among the active forms of immunotherapy recognized by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), should be mentioned the immunomodulatory mAbs, which mostly inhibit the immunosuppressive receptors expressed by activated T cells (e.g., Ipilimumab inhibiting CTLA-4 and Pembrolizumab inhibiting PD-1), the immunostimulatory cytokines, generally used as adjuvants of other anticancer immunotherapies (e.g., IL-2/Proleukin + Ipilimumab), the immunogenic cell death (ICD) inducers, which exert their antitumoral effect through cytostatic and cytotoxic mechanisms.
Immunomodulatory mAbs
In some pathological conditions, the so-called immune system checkpoints act directly as a “brake” in the immune response against cancer. The role of immunomodulatory monoclonal antibodies mAbs is precisely to lift these inhibitions by “removing the brake” of the immune system. To date, the most common and most widely used are the inhibitors of checkpoint Cytotoxic T Lymphocyte-Associated Antigen-4 (CTLA-4), Programmed Death 1 (PD-1) and PD-L1 [236]. There are six drugs targeting PD-1 or PDL-1 and only one targeting CTLA-4 currently approved for use in therapy of different types of cancer. Recently, the combination of two inhibitory checkpoints (i.e., ipilimumab (anti-CTLA-4 and nivolumab (anti-PD-1) has also joined the list of approved drugs, showing good therapeutic efficacy in several studies and thus paving the way for new clinical trials in different types of cancers [237]. However, other immune checkpoints are targeted in preliminary stages of clinical development [238]. In recent years, bispecific antibodies have also been developed with the aim of targeting multiple checkpoints simultaneously (e.g., CTLA-4 and PD-1), thus amplifying the signal [239]. However, this double-targeting has so far showed a higher toxicity as compared to its corresponding single therapies. Some of these double-targeting systems will be described in later sections of this review.
Immunostimulatory cytokines
With a counterbalancing action, immunostimulatory cytokines act instead as “stimulators” of the immune response. Cytokines can promote the activation, proliferation and survival of lymphocytes (T, B, NK) so as to obtain an antitumor response. Interleukins, interferons and chemokines belong to this large family. The protagonists in the field of immuno-oncology are certainly interleukin-2 (IL-2), the first cytokine FDA approved for therapeutic purposes, IL-12, -15, -21, and interferon alpha (IFN-ɑ), for a long time used for the treatment of hematological neoplasms, for renal carcinoma and melanoma [240]. Having a short half-life, the efficacy of these drugs is limited following their systemic administration. They also induce severe adverse effects before reaching therapeutic doses [241–243]. Today the new engineered generation of these cytokines is making its way into the world of oncological immunotherapy, with improved half-life, antitumor efficacy and toxicity [244].
Immunogenic cell death inducers
One of the most widely used ICD agents is Doxorubicin (DOX), a drug discovered in the late 1960s that acts as a DNA intercalating agent and induces apoptosis. As above-mentioned, it has been observed by several groups that some tumor cell lines express CD95L on their surface [245–249] and more importantly, that DOX-induced apoptosis is mediated by the expression of CD95L with the consequent induction of cell death by binding to CD95 [250]. This observation introduced a new perspective on the use of the targeted CD95/CD95L system. In the following years, several other cytotoxic drugs showed the ability to up-regulate the expression of CD95L in cancer cells. In addition to doxorubicin, among the many, we mention Cisplatin, Etoposide, 5-Fluorouracile, Methotrexate, and Bleomycin [251–253]. A parallel mechanism by which these DNA-damaging chemotherapy agents lead to autocrine or paracrine apoptosis of the cell involves the activation of the p53 system, which once activated acts as a transcription factor that regulates the expression of pro-apoptotic genes such as PUMA and BAX [254, 255]. Several studies have been conducted on the implication of p53 in the regulation of dose-dependent heavy side effects, the first of which is cardiotoxicity [256, 257]. Other studies explored the combination of DOX with different drugs with the aim to reduce both acute and chronic DOX-induced cardiotoxicity without affecting its p53-mediated anticancer activity. These studies mention beta-blockers (e.g., Carvedilol), iron-chelating agents (e.g., Dexrazoxane, DEX), angiotensin-converting enzyme inhibitors -ACEI- (e.g., Zofenopril) or even Flavonoids (e.g Frederine), used in combination with DOX for the attenuation of cardiotoxicity [258–261]. Very recently, Todorova et al. carried out a study in which a new combination of DOX and Dantrolene is proposed. Dantrolene appears to mitigate the cardiotoxicity of DOX without affecting its antitumor action in a breast cancer model [262]. Also in 2020, a team from China has developed a new method of co-administration of the DOX, according to which by pre-treating the triple-negative breast cancer cells MDA-MB-231 with Quercetin, followed by the DOX, it is possible to hinder the multidrug resistance of this aggressive cell line [263]. Taken together, these observations are promising for future development from a clinical point of view.
Cancer vaccines
The concept of cancer vaccines was first introduced in the 1990s when Bacillus Calmette-Guerin was approved by the FDA for the treatment of early-stage bladder cancer. To date, only three cancer vaccines have been approved by the FDA, due to the poor results often obtained in phases III and IV of the trials [264]. The cancer vaccine approach does not aim to prevent the cancer onset but to activate the immune system against cancer cells. The patent WO2015197874, published by a German team in 2017, proposes a combination of inhibition of the CD95/CD95L complex and cancer immunotherapy, such as a cancer vaccine [265–268]. As previously mentioned, CD95L expression on the tumor endothelium promotes an immunosuppressive environment through preferential killing of tumor-reactive CD8pos cells. Thus, the cancer vaccine would try to get the immune system to mount an attack against cancer cells by using a simultaneous inhibition of the CD95L/CD95 signaling system. More specifically, this cancer vaccine would contain cancer antigens in the form of a protein, a fragment thereof, or as RNA or DNA encoding that protein, to stimulate the immune system against this antigen.
Passive approaches
Monoclonal antibodies
Passive forms of immunotherapy include mAbs that specifically target the receptors on the surface of neoplastic cells expressing “tumor-associated antigens” (TAA), by altering their functions. Some of these antibodies can be administered in combination with chemotherapeutic agents so that the antibodies deliver these agents specifically to cancer cells. An example of such a system is represented by the combination of anti-CD95 antibodies with a chemotherapeutic agent such as 5-Fluorouracil or Tomudex (WO2003097698) [269, 355]. The goal is to synergize the pro-apoptotic effect of anti-CD95 mAbs with cancer chemotherapeutic agents to kill cancer cells. A different combination approach consists in genetically fusing a full-length monoclonal antibody targeting the cancer cell, such as rituximab, with a more biological component represented by a TNF superfamily ligand, such as CD95L, in its full-length, or truncated form, or a fragment thereof, thus offering two approaches to kill cancer cells. The antibody-TNFSF ligand fusion molecules would combine the specificity of the antibodies to the target antigen with the potent death-inducing properties of the TNFSF member ligand, thus providing improved efficacy and safety. The two combined killing approaches are thus performed through ADCC-independent apoptosis (Ab-dependent cellular cytotoxicity), and the second through the recruitment of effector cells to kill tumor targets (WO2012170072) [270]. Another technique involves the use of bispecific antibodies, the method of which consists of linking an antibody that reacts with the tumor cell to a second antibody that reacts with a cytotoxic effector cell. This is the case of patent WO2014076292 published by Baliopharm AG concerning a bispecific antibody with a first binding site for the CD95 receptor and a second binding site for the CD20 antigen [271, 272, 351]. This strategy aims to improve the treatment carried out with rituximab, an antibody able to target and kill CD20 expressing malignant and normal B cells unspecifically, thus showing significant side effects. This technique brings the effector cell into close opposition to the tumor cell, producing increased tumoricidal activity. Overall, the results of preclinical tests performed on antibody systems referring to CD95 have been encouraging, but to date, none of these CD95-related molecules are currently in clinical trials.
ACT adoptive cell transfer
Adoptive cell transfer is another very promising type of passive immunotherapy, which involves the re-introduction of specific effector cells into the patient bloodstream [273, 274]. Among them, Lymphokine Activated Killer Cells (LAK), Tumor-infiltrating lymphocytes (TILs) and Chimeric Antigen Receptors (CAR)-T cells are to be mentioned. Lymphokine Activated Killer Cells are obtained from the patient’s endogenous T cells, which are extracted, cultured in the presence of the lymphokine interleukin-2 (IL-2) and reinfused into the patient’s blood [275]. Tumor-infiltrating lymphocytes may have greater tumoricidal activity than LAKs, as they are isolated from resected tumor tissue, thus originating cells with greater tumor specificity than those obtained from blood [276]. An interesting method has recently been published by Iovance Biotherapeutics, Inc., concerning the expansion of TILs from tumor cells using, among others, CD95 agonists, for the treatment of diseases such as cancer (WO2018129332) [277, 278, 366]. A more recent strategy has been developed on the idea of genetically modifying T cells to express TAA-specific T-Cell Receptors, or Chimeric Antigen Receptors that recognize specific proteins on the cancer cells surface. Lately, it has been shown that CAR-T cells up-regulate the expression of CD95 and CD95L resulting in activation of the cell death program independently of TCR or CAR antigen-mediated activation [279]. The work of the Donda’s team highlights the importance of the role of the CD95/CD95L system in CAR-T cells-induced apoptosis by demonstrating the rescue of CAR-T cells upon in vivo blockade of this death-signaling pathway by CD95-Fc recombinant proteins. Patent US20180008670, published in 2018 in the U.S., concerns a method using CAR-T cells to stimulate immunity towards tumor endothelial cells. It is known that one of the limitations of CAR-T cells includes the lack of ability for the T cells to infiltrate deep into tumor tissue. In this formulation CAR-T cells would be able to destroy the CD95L-positive tumor endothelial cells, but also survive in their presence [280]. A year later an American group made the observation that CD95 is highly expressed on patient-derived T cells used for clinical ACT (adoptive cell transfer). They elaborated a T-cell co-engineered system including CD95 DNR (Dominant Negative Receptor) and either a T-cell receptor or Chimeric Antigen Receptor. These cells were genetically modified to express a defective CD95 variant, impairing the induction of apoptotic signal, together with a Chimeric Antigen Receptor, resulting in superior antitumor efficacy, greater longevity and no observed autoimmunity [281]. An interesting observation was recently made by Joshua D Brody’s team, who found that the CD95/CD95L system, in addition to its antigen-specific T-cell killing capability, mediates off-target “bystander” killing of antigen-negative tumor cells. They propose that CD95-mediated bystander elimination of Ag-loss variants may already be occurring in CAR-T treated patients. This process appears to be induced by CD95 upregulation on tumor cells after exposure to T-cell-secreted IFN-γ. They developed a CAR-T mouse model showing an improvement in tumor clearance when CD95 signaling is intact [282]. Overall, these observations open the door to promising new therapeutic opportunities exploiting the CD95/CD95L system in the cancer immunotherapy context
Therapeutic perspectives in cancer
It is well described that CD95 can promote pro-apoptotic and anti-apoptotic activities according to the physiological context [90]. Some previous studies have shown that down-regulating CD95 via shRNA in cancerous cells activates a death program by the induction of DNA damage and the activation of apoptotic effectors. One of these studies has been carried out and exposed in the US20100324116 patent, in which the inventors set out a siRNA-agent with the aim to reduce the amount of RNA encoding a CD95/CD95L gating polypeptides (e.g., FAPP2 or PATZ1 polypeptides) in significant quantities to sensitize brain tumor cells to CD95-mediated apoptosis [283, 284]. Over the past 25 years, several different potential therapeutic strategies related to the CD95/CD95L interaction have been studied. Among them, polypeptide systems, fusion proteins, methods, chemicals, antibodies and drug delivery systems are probably the most extensively studied.
Antitumoral polypeptides
Few patents describing CD95-related polypeptides have been published to provide a different approach in cancer treatment. In 2015 it was observed that blood polymorphonuclear neutrophils (PMNs) could kill cancer cells with a mechanism that remains to be elucidated [285]. Last year, a new method for reducing the toxicity of anticancer treatment on normal or non-cancerous cells has been registered as a patent at the University of Chicago (WO2020132465). This invention showed that ELANE, identified as the major anticancer protein released by PMNs, could cleave the CD95 receptor, releasing an intracellular proteolytic fragment containing the Death Domain and selectively killing a wide range of cancer cells [286]. The invention is a combination of specific CD95 peptides and the DNA encoding these peptides to treat different types of cancer. As previously mentioned, metalloproteases-cleaved CD95L (sCD95L) can exert a pro-oncogenic activity, through its interaction with CD95, promoting the survival and proliferation of cancer cells, but also their dissemination [141]. Therefore, a group proposed (WO2015158810) the use of polypeptides composed of the amino acid sequence encompassing the intracellular domain of CD95 which they previously identified as inducing a calcium-dependent cell motility process in T lymphocytes [145]. These inventors reported that the use of such peptides prevented the activation of PLCγ1 and the consequent calcium response that leads to cell migration. The same group reported a few years later that five molecules selected from the FDA/EMA-approved chemical library, namely Ritonavir, Diflunisal, Anethole, Rosiglitazone and Daunorubicin, could all block the recruitment of PLCγ1 to CD95 and reduce T lymphocyte motility (WO2018130679). Less recently, Wagner and Wei published a method related to the use of a combination of polypeptides and their encoding polynucleotides (WO2008067305) [287, 288]. They proposed a polypeptide composed of a ligand domain for a stimulatory Natural Killer receptor (e.g., the extracellular domain of MULT-I, which binds the NK cells receptor NKG2D) and the CD95 intracytoplasmic death domain. This method is supposed to activate the NK cells through the NKG2D receptor after contact with the tumor cells expressing the polypeptidic fusion compound so that not only the engaged tumor cells will be killed via CD95 induced-mechanisms but also are lysed directly by the activated NK cells.
CD95-related chimeric proteins
Another similar therapeutic approach related to the CD95/CD95L system for the treatment of cancer is the use of fusion proteins or chimeric proteins. Since the years 2000s, the fusion protein system has been perhaps the most widely studied. Patent WO2014121093 should be mentioned among the most recent of them [289]. Here, the inventors elaborated a chimeric system composed of a component capable of inducing the CD95-mediated apoptotic signal, and a component capable of blocking the CD47 receptor expressed at the tumor cell membrane and involved in the suppression of macrophage phagocytosis of the tumor cells.
The approach concerning fusion proteins that provide a physiologically similar oligomerized form of CD95L was studied by two different groups. One exposed a bi-component protein comprising the CTLA-4 extracellular domain and the CD95L extracellular domain, present in the form of a covalently bound and stable homo-hexamer, suitable for the treatment of a patient with cancer (WO2014106839) [290, 291]. If said patient has a tumor expressing the B7 receptor (e.g., B-cell lymphoma), this compound should be administered to exploit the double affinity of the bi-protein for the B7 and CD95 receptors, and finally inducing apoptosis of the malignant cells. The second group instead describes a chimeric protein composed of the extracellular domain of CD95L and a domain capable of inducing the oligomerization in this chimeric system (WO2013060864) [292–294]. Said domain is represented by the Ig-like domain of the Leukemia Inhibitory Factor (LIF) receptor gp190, which self-associates in the context of the chimeric protein giving rise to a dodecameric form with cytotoxic activity towards the cells expressing CD95. This system could therefore have various applications in the clinical field for the treatment of various diseases, such as cancer, autoimmune diseases and others.
Innovative antitumoral methods
The innovative methods approached in the context of the treatment of cancer patients are numerous and varied, among the most recent of which are the patents WO2015189236, WO2015104284, and WO2014118317, all filed and published by the same group. The first concerns a method aimed to reduce CD95-induced cell migration (WO2015189236). NHE1 is a NA+/H+ exchanger channel which this group reported to be indispensable for the CD95-induced cell motility process in fibroblasts [295]. This invention provides pharmaceutical compositions of compounds with NHE1 inhibiting properties to be administered if the subject shows elevated blood levels of sCD95L. This group also reported that in triple-negative breast cancer (TNBC), the serum level of CD95L could constitute an important parameter for the prognosis of the survival time and/or the relapse-free survival time. The same group therefore patented the invention WO2015104284, which aims to first determine the expression of sCD95L in the serum of subjects with triple-negative breast cancer (TNBC) and then to compare this level of expression to a predetermined standard value. The concluding step involves the administration to said subject of an effective therapeutic dose of plasma membranes structural components. The goal is to reduce the fluidity of the plasma membrane, a factor that this group reported as involved in the induction of cell migration by CD95 [296]. Subsequently, this same research group developed another patent, this time concerning the prediction and prevention of metastases in TNBC (WO2014118317). The authors describe a method for identifying serum levels of sCD95L in TNBC patients, stating that these patients develop a high risk of relapse if the level of sCD95L is significantly higher than a standard expression level [141]. In the same couple of years, another inventor published a method for predicting the sensitivity of tumor cells for a given treatment targeting inhibition of the CD95/CD95L system (WO2015107105) [297–299]. The invention more specifically concerns the analysis of the methylation levels of a DNA sequence of a gene belonging to this apoptotic signaling cascade obtained directly from a subject suffering from cancer, and consequent observation on the possible responsiveness of said cancer cells to a specific treatment. DNA and histone modifications remain the two major mechanisms of epigenetic regulation of gene expression [300]. Some inhibitors of these mechanisms, such as Decitabine and Vorinostat, are currently in clinical use to inhibit DNA methylation and histone acetylation respectively. An equally important role in the regulation of gene expression is played by the methylation of histone lysine residues through the action of Histone Methyltransferase (HMTase), for which to date only two chemical inhibitors (Verticillin A and Chaetocin) have been generated and found to be toxic in vivo. The Augusta University Research Institute, Inc. has developed a new inhibitor for HMTase SUV39H1 that appears to be useful in activating certain cytotoxic T-cell effectors, such as CD95L, thereby reversing cancer-induced immune suppression and promoting the killing of cancer cells by cytotoxic T cells (US20190084987) [301].
Currently used therapies and therapeutic perspectives in autoimmune diseases
Despite our growing knowledge of the immunological abnormalities that can lead to autoimmunity, the etiologies of most human autoimmune diseases remain unclear. This is probably because human autoimmune diseases are generally heterogeneous and multifactorial, not only between different diseases but also within the same disease [302]. They can, within a single disease, present a wide variety of clinical manifestations and severity, for instance the propagation speed, the number of affected joints, as well as a vast phenotypic heterogeneity. Besides, autoimmune diseases can clinically manifest long after the autoimmune reactions have been induced. Autoimmune diseases are often characterized by a severe imbalance between pro and anti-inflammatory mechanisms and by a vast diversity of signaling pathways and of cells and cytokines such as interleukins, interferons, and Treg cells that play a crucial role in immune tolerance. In recent decades, enormous progress has been made to identify the mechanisms associated with the activation and inactivation of T cells and to improve techniques based on the study of selective immune suppression in human autoimmune diseases. To date, the techniques used to counteract the mechanisms of autoimmunity are varied and include different peptide analogs, immunosuppressants, anti-inflammatories, monoclonal antibodies, inducers of immune tolerance, therapies targeting certain autoantigens, often used in conjunction with immunosuppressants to reduce their doses. Several groups around the world have carried out studies for which patents have been filed.
Multiple sclerosis
MS is a chronic autoimmune neurodegenerative disease that affects the central nervous system (CNS). It is characterized by an abnormal reaction of the immune defenses towards certain components of the CNS, damaging myelin and oligodendrocytes [303]. The symptoms are varied but CNS defective functions are frequent, with recurrent remissions and exacerbations. MS is suspected in patients with optic neuritis, especially if the deficits are multifocal or intermittent. In such cases, magnetic resonance imaging (MRI) scans of the brain and spinal cord and cerebrospinal fluid (CSF) analyses are performed, as this techniques allow to exclude other treatable pathologies that can mimic MS [304]. At the moment there is no definitive cure, but numerous therapies are available to modify its course, slowing its progression. The most severe form of MS is undoubtedly represented by relapsing-remitting multiple sclerosis (RRMS). Subjects with RRMS tend to have more brain lesions with widely varying localization and very different symptoms [305]. To date, the diagnosis to confirm the presence of the disease is given by tests resulting positive at least on two areas of myelin lesions in the CNS. These tests are not only painful but also risky and highly expensive. It is, therefore, necessary to develop additional methods for the diagnosis of this disease. The inventors of US20160194714 offer a new method for detecting relapse in RRMS patients using biomarkers, such as CD95L, sirtuin 1 (SIRT1), RGC-32 and IL-21, in a population of cells (e.g., PBMCs, CD4pos, CD8pos, glial cells, neurons, etc.) [306–309]. They noted a decrease in CD95L, SIRT1, and RGC-32 in relapsing RRMS patients, while an increase in IL-21 occurs. Overall, these four proteins can be used as markers to highlight the activity of this disease.
Systemic lupus erythematosus
The involvement of CD95L has been extensively studied in different chronic inflammatory autoimmune diseases, such as MS, SLE and RA. Several groups have observed differences in the frequency of the T-helper cells (Th) subgroups in SLE patients versus HCs (Healthy Controls), which also differ in their sensitivity to TCR-mediated cell death [310–313]. This could explain the discordant results on CD95L expression levels in total lymphocytes from healthy donors and patients with chronic inflammatory disease. A few years ago, it was noted that transcription of CD95L is a crucial step for the regulation of T-helper cell death sensitivity. This group found that human Th1 cells express higher mRNA levels of CD95L than Th17 cells. Resistance of Th17 cells to AICD was associated with lower expression of CD95L and overexpression of the anti-apoptotic caspase-8 inhibitory protein (FLIP) [314]. In the mid-2000s, an important role was attributed to these IL-17A and IL-17F producing lymphocytes in the context of autoimmune diseases. Th17 cells orchestrate autoimmune inflammation, in addition to their function as eliminators of extracellular pathogens [315–317]. Yang et al. observed that SLE patients exhibit significant infiltration of Th17 lymphocytes secreting cytokines in their skin [318]. It is therefore possible to hypothesize that by modulating their trafficking to the organs, the pathogenesis of the SLE disease could consequently be modulated. In the context of this chronic inflammatory disease, soluble CD95L (sCD95L) has been shown to be involved in promoting the trafficking of Th17 lymphocytes into damaged organs, at the expense of Treg lymphocytes in a CD95-driven murine model of SLE [142]. Blocking the CD95/CD95L system could thus represent an attractive approach for the treatment of Th17 cell-mediated diseases. This was the intent of the authors of the WO2016170027 patent, who proposed to use CD95 antagonist antibodies, having specificity for CD95 or sCD95L, with the potential to prevent the endothelial transmigration of Th17 cells in the organs and the consequent damage given by the accumulation of the activated T cells in said organs [142]. DR-mediated cell death is essential for the differentiation, growth and function of lymphocytes. In 2017, Croft and Siegel discussed the implication of some of these receptors in inducing inflammation and their potential in future therapies for rheumatoid diseases [319]. Interestingly, the combined blockade of TNFR1, TRAIL-R and CD95 seems to give excellent results in the prevention of inflammation caused by the respective ligands, whereas targeting these receptors individually did not have that effect (WO2019141862) as demonstrated in a murine model of dermatitis [320]. Such observations lead to the conclusion that different cell DR systems may act in combination to contribute to the pathogenesis of autoimmune inflammatory diseases. Importantly, uncontrolled induction of cell death downstream of DR, rather than increased DR-induced gene-activatory signaling pathways, could actually be key in driving inflammation in such contexts [320–322]. Interestingly, the Decoy Receptor 3 (DcR3), encoded by the TNFRSF6B gene, was found to act as a regulator of the amplification of the immune response by binding with stimulatory cytokines, such as CD95L, TL1A and LIGHT, limiting the interaction of the latter with their own receptor [323]. It, therefore, seems deductible that genetic modifications of the TNFRSF6B gene, involving a reduced expression of DcR3, or a lower binding activity for the aforementioned cytokine, or even the suppression of its expression, could contribute to cause inflammatory signals. With this idea in view, the inventors of US20170051352 have developed a method for treating autoimmune conditions in patients carrying alterations of the gene encoding the DcR3 protein, or of a DcR3 network gene, by administering to said patient an effective amount of DcR3 ligands inhibitors [323].
Fusion proteins in the context of autoimmune diseases
As in the context of cancer, one of the widely adopted strategies in studying new potential treatments for autoimmune diseases is represented by the use of fusion proteins and nucleotides that encode them. In 2018, APOGENIX AG published a patent relating to a nucleotide sequence encoding an isolated chimeric compound formed by the extracellular domain of CD95 and an immunoglobulin domain or a functional fragment thereof. The inventors intend to generate a stable system to inhibit the extrinsic apoptotic signal initiated by CD95L for the prophylaxis or treatment of various diseases, including autoimmune diseases and solid cancers (US20180186856) [324, 325, 402]. A few years earlier the same inventors developed a mixture of fusion protein isoforms having the same composition as the aforementioned system with the difference that this patent does not mention any nucleotide sequence encoding the chimeric protein, as well as the cell hosting the nucleotide sequence (WO2014013039). A different chimeric system is represented by the invention WO2016205714, which exposes an immune tolerance inducer “medicament” comprising a CD95L moiety together with a streptavidin or avidin moiety [326–328]. The claimed compound is to be administered alone or mixed with the IL-2 protein to achieve sequential or simultaneous action in inducing long-term and specific immunosuppression. CD95L is then part of another fusion compound, the one described by the patent WO2014121085, in which the extracellular domain of CD95L corresponds to half of the fusion protein. The other half is the extracellular domain of a PD-1 receptor-activating factor, such as its ligand PD-L1 and PD-L2 [329]. This system aims to inhibit the differentiation and proliferation of a selection of cells, including activated T cells on which the PD-1 receptor is widely expressed, thus the induction of PD-1 ligation by its ligands mediates an inhibitory signal that results in reduced cytokine production and reduced T-cell survival. Thus, in the setting of autoimmune and inflammatory diseases, the fusion protein of this invention could reduce autoimmune and inflammatory manifestations.
Cells engineering modifications
Some other groups have then explored the field of cells engineering modification by proposing methods of isolating these cells from a patient sample, treating/modifying these cells and reintroducing the said modified cells by systemic infusion or transplantation. This is the case of the patent WO2013149211, which describes a method using modified mesenchymal stem cells (MSCs) to overexpress CD95, CD95L as well as the CD95-regulated monocyte chemotactic protein 1 (MCP-1) which seems to play an important role in the recruitment of T cells to MSCs [330, 331]. It has previously been hypothesized that such MSCs play an important role in reducing T-cell proliferation through a mechanism involving T-cell apoptosis [332]. Therefore, this invention offers a potential therapeutic method for the treatment of autoimmune diseases, and more specifically of Systemic Sclerosis. Similarly, patent WO2015038665 relates to a system composed of modified MSCs to overexpress CD95L after exposure of these cells to a salicylate, such as common aspirin [333]. The authors offer a method aimed at increasing survival rates in patients suffering from autoimmune and inflammatory diseases. In 2016, another modified cell-related strategy was developed by the Trustees of the University of Pennsylvania, which involves the use of genetically modified effector cells to downregulate endogenous CD95 using the CRISPR system to treat autoimmune diseases (WO2016069282) [364].
Discussion and conclusion
For nearly three decades, members of the TNF superfamily, and the signal cascades they trigger, have been targeted by researchers and pharmaceutical companies to develop new therapies for the treatment of cancer and autoimmune diseases [334–338]. These molecules are widely involved in multiple cellular mechanisms such as apoptosis, proliferation, survival, tumor growth and differentiation. Since their role in mediating immune surveillance as well as protection from infections is essential, prolonged inhibition of these molecules could be dangerous. The progenitor of the TNF superfamily (i.e., TNF) remains the most studied and the most promising in terms of therapeutic potential [337, 338]. Among the members of the TNF superfamily, the research carried out on the TNF system is the most funded, with sales revenues exceeding 25 billion USD [338] followed by DR4/DR5 (Trail) systems and finally by the CD95 complex. Currently, five anti-TNF biologics have been clinically approved for the treatment of autoimmune diseases, namely Infliximab, Adalimumab, Etanercept, Golimumab, and Certolizumab Pegol, all with a specific structure for TNF-alpha recognition and blockade [339]. Despite the evident efficacy of these drugs, not all treated patients respond as expected and some seem to develop adverse reactions associated with these drugs, such as effects on the neurological and dermatological levels [340–342]. There is therefore a growing need for new pharmacological systems with better specificity and greater safety.
CD95-related therapeutic perspectives
Despite the evident role of CD95/CD95L in cancer and chronic inflammatory autoimmune diseases, since 1990 only a little over a hundred patents targeting the CD95/CD95L system have been conceived and published (Fig. 4). In the past, the complexity of the multiple CD95/CD95L-mediated signaling systems found in cancer and autoimmune diseases, the lack of specificity of the previously proposed strategies tested in vivo and the consequent severe side effects found [219, 220], have diminished the pharmacological interest for this target. Recent study report strategies focusing on more challenging compounds and delivery methods, with a particular attention to circumventing the severe adverse effects associated with the systemic activation of CD95. The extensively studied CD95-Fc fusion proteins, for instance, represent an interesting way to inhibit CD95L. However, these chimeric proteins, compared to those used in the TNF-TNFR2 system [343], exhibit a relatively low affinity for the corresponding CD95L and far less efficacy in inhibiting death induced by ligand interaction with CD95. A possible explanation is given by the fact that the interactions between these proteins occur through a complex mechanism of oligomerization given by the association of multiple trimers of both counterparts [35, 294, 344]. A better neutralization or stimulation of these proteins might therefore be achieved by a neutralization/stimulation system in which the binding protein is in a stable physiological-like form consisting of at least one trimer, if not an oligomer thereof. It seems that the oligomerization of the binding protein improves the stability of the therapeutic compound, consequently increasing its affinity for the target and the final system specificity [345]. Such oligomerized-related strategies have exhibited more efficient results, compared to previous systems generations. Fortunately, some of the newly proposed strategies appear to give encouraging preclinical results and so far, only one of these is currently in clinical trials. APG101 is the best prototype of future therapeutic approaches involving the CD95 system. It is an 84 kDa CD95L-neutralizing CD95 trimer fusion protein, able to pass the blood-brain barrier. Asunercept, the trade name for APG101, is now the subject of a controlled phase II clinical trial in patients with relapsed glioblastoma multiforme (GBM) (NCT01071837). The glioblastoma model was chosen in accordance with several in vivo and in vitro non-clinical studies, which extensively described the involvement of CD95L in the growth, invasiveness and migration of glioblastoma cells [152, 324]. Merz et al. observed a decreased invasiveness on two cellular models of GBM after knockdown of the FASLG gene, without however affecting the viability of the cells sensitive to apoptosis [325]. They also reported a restored invasiveness following the administration of soluble recombinant CD95L, which was blocked by the addition of the APG101 fusion protein. This formulation, consisting of the extracellular domain of human CD95 and the Fc domain of human IgG1, was in fact designed to specifically bind CD95L, thus disrupting the CD95L/CD95 signal cascade and the resulting cellular invasiveness. The collected results show a remarkable survival prolongation in patients with GBM, which makes it interesting for a possible transfer to other types of cancer [324, 346]. Furthermore, some experiments carried out on a cohort of 84 patients, showed greater efficacy of this compound when it is administered in combination with radiotherapy, observing a significant reduction in tumor growth compared to radiotherapy treatment alone [347]. Other preclinical studies, conducted on patients suffering from a lower risk myelodysplastic syndrome (MDS), have then highlighted a possible role of Asunercept in the treatment of anemia, a characteristic feature of this pathological condition [348]. In low risk MDS the administration of erythropoiesis-stimulating agents (ESAs) is widely used to correct cytopenia. However, some patients show resistance to ESA, thus requiring alternative treatments to contain the anemia associated with low risk MDS. CD95 is overexpressed in two-thirds of MDS patients, and is thought to be negatively implicated in the regulation of erythrocyte production [348]. The blocking of CD95 signal cascade can therefore increase erythropoiesis in MDS patients. The use of APG101 in this context seems to be particularly promising, as the neutralization of CD95L allows the blocking of the CD95L/CD95L signal and finally the restoration of erythropoiesis. A phase I clinical study (NCT01736436) conducted on 20 patients with low and intermediate MDS treated with intravenous APG101 is currently underway [349]. In said patients APG101 showed good tolerance and safety, promising prerequisites for use on a larger scale of this drug in the future.
Fig. 4. Contribution of the CD95L/CD95 system in therapeutic-end studies.
Graphic representation of the distribution of the number of patents targeting the most studied cell Death Receptors CD95, TNFR1 and -R2, TRAILR1 and -R2 and their respective ligands CD95L, TNF alpha, and TRAIL.
Supplementary information
Acknowledgements
Figures 1–3 made use of graphic elements modified from the Servier Medical Art website (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License (https://creativecommons.org/licenses/by/3.0/).
Author contributions
Conceptualization, VR and MLG; Wrote the original draft, collected the literature VR; Writing—review and editing MLG and EL.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 777995, from ANR (FASILE), ARC (PJA20181207700), Fondation de France and association La Vannetaise.
Data availability
All data generated or analyzed during this study are included in this published article and Supplementary Materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-022-04688-x.
References
- 1.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192:F21–5. [PubMed] [Google Scholar]
- 6.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 8.Moldoveanu T, Czabotar PE. BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harbor Pers Biol. 2020;12. 10.1101/CSHPERSPECT.A036319. [DOI] [PMC free article] [PubMed]
- 9.Sandow JJ, Tan IK, Huang AS, Masaldan S, Bernardini JP, Wardak AZ, et al. Dynamic reconfiguration of pro-apoptotic BAK on membranes. EMBO J. 2021;40. 10.15252/EMBJ.2020107237. [DOI] [PMC free article] [PubMed]
- 10.Birkinshaw RW, Iyer S, Lio D, Luo CS, Brouwer JM, Miller MS, et al. Structure of detergent-activated BAK dimers derived from the inert monomer. Mol Cell. 2021;81:2123–34.e5. doi: 10.1016/j.molcel.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Sperl LE, Rührnößl F, Schiller A, Haslbeck M, Hagn F. High-resolution analysis of the conformational transition of pro-apoptotic Bak at the lipid membrane. EMBO J. 2021;40. 10.15252/EMBJ.2020107159. [DOI] [PMC free article] [PubMed]
- 12.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–13. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 13.Dorstyn L, Akey CW, Kumar S. New insights into apoptosome structure and function. Cell Death Differ. 2018;25:1194–208. doi: 10.1038/s41418-017-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–9. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 17.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 19.Abbas R, Larisch S. Targeting XIAP for promoting cancer cell death—the story of ARTS and SMAC. Cells 2020;9. 10.3390/CELLS9030663. [DOI] [PMC free article] [PubMed]
- 20.van Loo G, van Gurp M, Depuydt B, Srinivasula SM, Rodriguez I, Alnemri ES, et al. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002;9:20–6. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–21. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 22.Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM, et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem. 2002;277:445–54. doi: 10.1074/jbc.M109891200. [DOI] [PubMed] [Google Scholar]
- 23.Miguel Martins L, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, et al. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J Biol Chem. 2002;277:439–44. doi: 10.1074/jbc.M109784200. [DOI] [PubMed] [Google Scholar]
- 24.Barillé-Nion S, Lohard S, Juin PP. Targeting of BCL-2 family members during anticancer treatment: a necessary compromise between individual cell and ecosystemic responses? Biomolecules. 2020;10:1–24.. doi: 10.3390/biom10081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero J, Sarosiek KA, Deangelo JD, Maertens O, Ryan J, Ercan D, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–89. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Certo M, Moore VDG, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–97. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Tait SWG, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Muñoz-Pinedo C, et al. Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev Cell. 2010;18:802–13. doi: 10.1016/j.devcel.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell. 2015;57:860–72. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rongvaux A, Jackson R, Harman CCD, Li T, West AP, De Zoete MR, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–77. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giampazolias E, Zunino B, Dhayade S, Bock F, Cloix C, Cao K, et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat Cell Biol. 2017;19:1116–29. doi: 10.1038/ncb3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diepstraten ST, Anderson MA, Czabotar PE, Lessene G, Strasser A, Kelly GL. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer. 2021. 10.1038/S41568-021-00407-4. [DOI] [PubMed]
- 34.Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E. The death domain: a module shared by proteins with diverse cellular functions. Trends Biochem Sci. 1995;20:342–4. doi: 10.1016/s0968-0004(00)89070-2. [DOI] [PubMed] [Google Scholar]
- 35.Papoff G, Hausler P, Eramo A, Pagano MG, Leve GDI, Signore A, et al. Identification and characterization of a ligand-independent oligomerization domain in the extracellular region of the CD95 death receptor. J Biol Chem. 1999;274:38241–50. doi: 10.1074/jbc.274.53.38241. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RM, Frederiksen JK, Zacharias DA, Chan FKM, Johnson M, Lynch D, et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–7. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 37.Steller EJA, Borel Rinkes IHM, Kranenburg O. How CD95 stimulates invasion. Cell Cycle. 2011;10:3857–62. doi: 10.4161/cc.10.22.18290. [DOI] [PubMed] [Google Scholar]
- 38.Berg D, Lehne M, Müller N, Siegmund D, Münkel S, Sebald W, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 2007;14:2021–34. doi: 10.1038/sj.cdd.4402213. [DOI] [PubMed] [Google Scholar]
- 39.Sun M, Ames KT, Suzuki I, Fink PJ. The cytoplasmic domain of fas ligand costimulates TCR signals. J Immunol. 2006;177:1481–91. doi: 10.4049/jimmunol.177.3.1481. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J Immunol. 2000;165:5537–43. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- 41.Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat Med. 1998;4:1377–82. doi: 10.1038/3965. [DOI] [PubMed] [Google Scholar]
- 42.Paulsen M, Mathew B, Qian J, Lettau M, Kabelitz D, Janssen O. FasL cross-linking inhibits activation of human peripheral T cells. Int Immunol. 2009;21:587–98. doi: 10.1093/intimm/dxp028. [DOI] [PubMed] [Google Scholar]
- 43.Fu Q, Fu TM, Cruz AC, Sengupta P, Thomas SK, Wang S, et al. Structural basis and functional role of intramembrane trimerization of the Fas/CD95 death receptor. Mol Cell. 2016;61:602–13. doi: 10.1016/j.molcel.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, et al. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature. 2009;457:1019–22. doi: 10.1038/nature07606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, et al. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–44. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahdizadeh SJ, Thomas M, Eriksson LA. Reconstruction of the fas-based death-inducing signaling complex (DISC) using a protein-protein docking meta-approach. J Chem Inf Model. 2021;61:3543–58. doi: 10.1021/acs.jcim.1c00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinnaiyan AM, Tepper CG, Seldin MF, O’Rourke K, Kischkel FC, Hellbardt S, et al. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–5. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 49.Yeh WC, De La Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 50.Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, et al. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–53. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickens LS, Boyd RS, Jukes-Jones R, Hughes MA, Robinson GL, Fairall L, et al. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol Cell. 2012;47:291–305. doi: 10.1016/j.molcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schleich K, Warnken U, Fricker N, Öztürk S, Richter P, Kammerer K, et al. Stoichiometry of the CD95 death-inducing signaling complex: experimental and modeling evidence for a death effector domain chain model. Mol Cell. 2012;47:306–19. doi: 10.1016/j.molcel.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Fu TM, Li Y, Lu A, Li Z, Vajjhala PR, Cruz AC, et al. Cryo-EM structure of caspase-8 tandem DED filament reveals assembly and regulation mechanisms of the death-inducing signaling complex. Mol Cell. 2016;64:236–50. doi: 10.1016/j.molcel.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox JL, Hughes MA, Meng X, Sarnowska NA, Powley IR, Jukes-Jones R, et al. Cryo-EM structural analysis of FADD:Caspase-8 complexes defines the catalytic dimer architecture for co-ordinated control of cell fate. Nat Commun. 2021;12. 10.1038/S41467-020-20806-9. [DOI] [PMC free article] [PubMed]
- 55.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Lee SJ, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–9. doi: 10.1038/s41586-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 59.Pop C, Fitzgerald P, Green DR, Salvesen GS. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46:4398–407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 60.Chang DW, Xing Z, Capacio VL, Peter ME, Yang X. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22:4132–42. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tummers B, Green DR. Caspase-8: regulating life and death. Immunological Rev. 2017;277:76–89. doi: 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majkut J, Sgobba M, Holohan C, Crawford N, Logan AE, Kerr E, et al. Differential affinity of FLIP and procaspase 8 for FADD’s DED binding surfaces regulates DISC assembly. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes MA, Powley IR, Jukes-Jones R, Horn S, Feoktistova M, Fairall L, et al. Co-operative and Hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell. 2016;61:834–49. doi: 10.1016/j.molcel.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavrik I, Krueger A, Schmitz I, Baumann S, Weyd H, Krammer PH, et al. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death Differ. 2003;10:144–5. doi: 10.1038/sj.cdd.4401156. [DOI] [PubMed] [Google Scholar]
- 65.Schleich K, Buchbinder JH, Pietkiewicz S, Kähne T, Warnken U, Öztürk S, et al. Molecular architecture of the DED chains at the DISC: regulation of procaspase-8 activation by short DED proteins c-FLIP and procaspase-8 prodomain. Cell Death Differ. 2015;23:681–94. doi: 10.1038/cdd.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–14. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu JW, Jeffrey PD, Shi Y. Mechanism of procaspase-8 activation by c-FLIPL. Proc Natl Acad Sci USA. 2009;106:8169–74. doi: 10.1073/pnas.0812453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–71. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 69.Humphreys LM, Fox JP, Higgins CA, Majkut J, Sessler T, McLaughlin K, et al. A revised model of TRAIL-R2 DISC assembly explains how FLIP(L) can inhibit or promote apoptosis. EMBO Rep. 2020;21. 10.15252/EMBR.201949254. [DOI] [PMC free article] [PubMed]
- 70.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–7. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 72.Sprick MR, Rieser E, Stahl H, Grosse-Wilde A, Weigand MA, Walczak H. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 2002;21:4520–30. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakamaki K, Imai K, Tomii K, Miller DJ. Evolutionary analyses of caspase-8 and its paralogs: deep origins of the apoptotic signaling pathways. BioEssays. 2015;37:767–76. doi: 10.1002/bies.201500010. [DOI] [PubMed] [Google Scholar]
- 74.Fischer U, Stroh C, Schulze-Osthoff K. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene. 2006;25:152–9. doi: 10.1038/sj.onc.1209015. [DOI] [PubMed] [Google Scholar]
- 75.Lafont E, Milhas D, Teissié J, Therville N, Andrieu-Abadie N, Levade T, et al. Caspase-10-dependent cell death in Fas/CD95 signalling is not abrogated by caspase inhibitor zVAD-fmk. PloS ONE. 2010;5. 10.1371/JOURNAL.PONE.0013638. [DOI] [PMC free article] [PubMed]
- 76.Backus KM, Correia BE, Lum KM, Forli S, Horning BD, González-Páez GE, et al. Proteome-wide covalent ligand discovery in native biological systems. Nature. 2016;534:570–4. doi: 10.1038/nature18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milhas D, Cuvillier O, Therville N, Clavé P, Thomsen M, Levade T, et al. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem. 2005;280:19836–42. doi: 10.1074/jbc.M414358200. [DOI] [PubMed] [Google Scholar]
- 78.Engels IH, Totzke G, Fischer U, Schulze-Osthoff K, Jänicke RU. Caspase-10 sensitizes breast carcinoma cells to TRAIL-induced but not tumor necrosis factor-induced apoptosis in a caspase-3-dependent manner. Mol Cell Biol. 2005;25:2808–18. doi: 10.1128/MCB.25.7.2808-2818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98:13884–8. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horn S, Hughes MA, Schilling R, Sticht C, Tenev T, Ploesser M, et al. Caspase-10 negatively regulates caspase-8-mediated cell death, switching the response to CD95L in favor of NF-κB activation and cell survival. Cell Rep. 2017;19:785–97. doi: 10.1016/j.celrep.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mühlethaler-Mottet A, Flahaut M, Balmas Bourloud K, Nardou K, Coulon A, Liberman J, et al. Individual caspase-10 isoforms play distinct and opposing roles in the initiation of death receptor-mediated tumour cell apoptosis. Cell Death Dis. 2011;2. 10.1038/CDDIS.2011.8. [DOI] [PMC free article] [PubMed]
- 82.Seyrek K, Ivanisenko NV, Richter M, Hillert LK, König C, Lavrik IN. Controlling cell death through post-translational modifications of DED proteins. Trends Cell Biol. 2020;30:354–69. doi: 10.1016/j.tcb.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Seyrek K, Richter M, Lavrik IN. Decoding the sweet regulation of apoptosis: the role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019;26:981–93. doi: 10.1038/s41418-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lafont E, Hartwig T, Walczak H. Paving TRAIL’s path with ubiquitin. Trends Biochem Sci. 2018;43:44–60. doi: 10.1016/j.tibs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DCS, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–9. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson TR, McEwan M, McLaughlin K, Le Clorennec C, Allen WL, Fennell DA, et al. Combined inhibition of FLIP and XIAP induces Bax-independent apoptosis in type II colorectal cancer cells. Oncogene. 2009;28:63–72. doi: 10.1038/onc.2008.366. [DOI] [PubMed] [Google Scholar]
- 88.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465:492–6. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peter ME, Hadji A, Murmann AE, Brockway S, Putzbach W, Pattanayak A, et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015;22:549–59. doi: 10.1038/cdd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin-Villalba A, Llorens-Bobadilla E, Wollny D. CD95 in cancer: tool or target? Trends Mol Med. 2013;19:329–35. doi: 10.1016/j.molmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Peter ME, Legembre P, Barnhart BC. Does CD95 have tumor promoting activities? Biochim Biophys Acta. 2005;1755:25–36. doi: 10.1016/j.bbcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Tang D, Kang R, Berghe TVanden, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 94.Ivanov VN, Bergami PL, Maulit G, Sato T-A, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–35. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–52. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin MS, Park WS, Kim SY, Kim HS, Kang SJ, Song KY, et al. Alterations of Fas (Apo-1/CD95) gene in cutaneous malignant melanoma. Am J Pathol. 1999;154:1785–91. doi: 10.1016/S0002-9440(10)65434-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SH, Shin MS, Park WS, Kim SY, Kim HS, Han JY, et al. Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene. 1999;18:3754–60. doi: 10.1038/sj.onc.1202769. [DOI] [PubMed] [Google Scholar]
- 98.Lee S, Shin M, Park W, Kim S, Dong S, Pi J, et al. Alterations of Fas (APO-1/CD95) gene in transitional cell carcinomas of urinary bladder. Cancer Res. 1999;59:3068–72. [PubMed] [Google Scholar]
- 99.Park S, Oh R, Kim YS, Park Y, Lee H, Shin MS, et al. Somatic mutations in the death domain of the Fas (Apo-1/CD95) gene in gastric cancer. J Pathol. 2001;193:162–8. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path759>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 100.Tauzin S, Debure L, Moreau JF, Legembre P. CD95-mediated cell signaling in cancer: mutations and post-translational modulations. Cell Mol Life Sci. 2012;69:1261–77. doi: 10.1007/s00018-011-0866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poissonnier A, Guégan J-P, Nguyen HT, Best D, Levoin N, Kozlov G, et al. Disrupting the CD95–PLCγ1 interaction prevents Th17-driven inflammation. Nat Chem Biol. 2018;14:1079–89. doi: 10.1038/s41589-018-0162-9. [DOI] [PubMed] [Google Scholar]
- 102.Martin DA, Zheng L, Siegel RM, Huang B, Fisher GH, Wang J, et al. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci. 1999;96:4552–7. doi: 10.1073/pnas.96.8.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ta NL, Chakrabandhu K, Huault S, Hueber AO. The tyrosine phosphorylated pro-survival form of Fas intensifies the EGF-induced signal in colorectal cancer cells through the nuclear EGFR/STAT3-mediated pathway. Sci Rep. 2018;8:12424. doi: 10.1038/s41598-018-30804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, et al. Fas/CD95-induced chemokines can serve as ‘find-me’ signals for apoptotic cells. Mol Cell. 2013;49:1034–48. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 105.Neumann L, Pforr C, Beaudouin J, Pappa A, Fricker N, Krammer PH, et al. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells. Mol Syst Biol. 2010;6. 10.1038/MSB.2010.6. [DOI] [PMC free article] [PubMed]
- 106.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buchbinder JH, Pischel D, Sundmacher K, Flassig RJ, Lavrik IN. Quantitative single cell analysis uncovers the life/death decision in CD95 network. PLoS Comput Biol. 2018;14. 10.1371/JOURNAL.PCBI.1006368. [DOI] [PMC free article] [PubMed]
- 108.Schmidt JH, Pietkiewicz S, Naumann M, Lavrik IN. Quantification of CD95-induced apoptosis and NF-κB activation at the single cell level. J Immunol Methods. 2015;423:12–17. doi: 10.1016/j.jim.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 109.Kreuz S, Siegmund D, Rumpf JJ, Samel D, Leverkus M, Janssen O, et al. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol. 2004;166:369–80. doi: 10.1083/jcb.200401036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Imamura R, Konaka K, Matsumoto N, Hasegawa M, Fukui M, Mukaida N, et al. Fas ligand induces cell-autonomous NF-kappaB activation and interleukin-8 production by a mechanism distinct from that of tumor necrosis factor-alpha. J Biol Chem. 2004;279:46415–23. doi: 10.1074/jbc.M403226200. [DOI] [PubMed] [Google Scholar]
- 111.Matsuda I, Matsuo K, Matsushita Y, Haruna Y, Niwa M, Kataoka T. The C-terminal domain of the long form of cellular FLICE-inhibitory protein (c-FLIPL) inhibits the interaction of the caspase 8 prodomain with the receptor-interacting protein 1 (RIP1) death domain and regulates caspase 8-dependent nuclear factor κB (NF-κB) activation. J Biol Chem. 2014;289:3876–87. doi: 10.1074/jbc.M113.506485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, et al. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–66. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- 113.Henry CM, Martin SJ. Caspase-8 acts in a non-enzymatic role as a scaffold for assembly of a pro-inflammatory ‘FADDosome’ complex upon TRAIL stimulation. Mol Cell. 2017;65:715–29.e5. doi: 10.1016/j.molcel.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 114.Lafont E, Kantari‐Mimoun C, Draber P, De Miguel D, Hartwig T, Reichert M, et al. The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. EMBO J. 2017;36:1147–66. doi: 10.15252/embj.201695699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trauzold A, Röder C, Sipos B, Karsten K, Arlt A, Jiang P, et al. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J. 2005;19:1–24. doi: 10.1096/fj.04-2984fje. [DOI] [PubMed] [Google Scholar]
- 116.Shimizu Y, Peltzer N, Sevko A, Lafont E, Sarr A, Draberova H, et al. The Linear ubiquitin chain assembly complex acts as a liver tumor suppressor and inhibits hepatocyte apoptosis and hepatitis. Hepatology. 2017;65:1963–78. doi: 10.1002/hep.29074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turner DJ, Alaish SM, Zou T, Rao JN, Wang J-Y, Strauch ED. Bile salts induce resistance to apoptosis through NF-κB-mediated XIAP expression. Ann Surg. 2007;245:415. doi: 10.1097/01.sla.0000236631.72698.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jönsson G, Paulie S, Grandien A. cIAP-2 block apoptotic events in bladder cancer cells. Anticancer Res. 2003;23:3311–6. [PubMed] [Google Scholar]
- 119.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–73. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shinohara H, Yagita H, Ikawa Y, Oyaizu N. Fas drives cell cycle progression in glioma cells via extracellular signal-regulated kinase activation. Cancer Res. 2000;60:1766–72. [PubMed] [Google Scholar]
- 122.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 123.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krens SFG, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006;580:4984–90. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 125.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–76. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu R, Hu J. The role of JNK in prostate cancer progression and therapeutic strategies. Biomedicine Pharmacother. 2020;121:109679. doi: 10.1016/j.biopha.2019.109679. [DOI] [PubMed] [Google Scholar]
- 127.Kim YY, Park BJ, Seo GJ, Lim JY, Lee SM, Kimm KC, et al. Long form of cellular FLICE-inhibitory protein interacts with Daxx and prevents Fas-induced JNK activation. Biochem Biophys Res Commun. 2003;312:426–33. doi: 10.1016/j.bbrc.2003.10.144. [DOI] [PubMed] [Google Scholar]
- 128.Deak JC, Cross JV, Lewis M, Qian Y, Parrott LA, Distelhorst CW, et al. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA. 1998;95:5595–600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kavuri SM, Geserick P, Berg D, Dimitrova DP, Feoktistova M, Siegmund D, et al. Cellular FLICE-inhibitory protein (cFLIP) isoforms block CD95- and TRAIL death receptor-induced gene induction irrespective of processing of caspase-8 or cFLIP in the death-inducing signaling complex. J Biol Chem. 2011;286:16631–46. doi: 10.1074/jbc.M110.148585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J, et al. Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem. 2001;276:47100–6. doi: 10.1074/jbc.M107385200. [DOI] [PubMed] [Google Scholar]
- 131.Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. 2003;5:118–25. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 132.Siegmund D, Klose S, Zhou D, Baumann B, Röder C, Kalthoff H, et al. Role of caspases in CD95L- and TRAIL-induced non-apoptotic signalling in pancreatic tumour cells. Cell Signal. 2007;19:1172–84. doi: 10.1016/j.cellsig.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 133.Farley SM, Purdy DE, Ryabinina OP, Schneider P, Magun BE, Iordanov MS. Fas ligand-induced proinflammatory transcriptional responses in reconstructed human epidermis. Recruitment of the epidermal growth factor receptor and activation of MAP kinases. J Biol Chem. 2008;283:919–28. doi: 10.1074/jbc.M705852200. [DOI] [PubMed] [Google Scholar]
- 134.Barca O, Seoane M, Señarís MR, Arce VM. Fas/CD95 ligation induces proliferation of primary fetal astrocytes through a mechanism involving caspase 8-mediated ERK activation. Cell Physiol Biochem. 2013;32:111–20. doi: 10.1159/000350129. [DOI] [PubMed] [Google Scholar]
- 135.Koenig A, Buskiewicz IA, Fortner KA, Russell JQ, Asaoka T, He YW, et al. The c-FLIPL cleavage product p43FLIP promotes activation of extracellular signal-regulated kinase (ERK), nuclear factor κB (NF-κB), and caspase-8 and T cell survival. J Biol Chem. 2014;289:1183–91. doi: 10.1074/jbc.M113.506428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys. 2002;408:155–61. doi: 10.1016/s0003-9861(02)00525-8. [DOI] [PubMed] [Google Scholar]
- 137.Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, et al. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2007;205:74–81. doi: 10.1016/j.expneurol.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 138.Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22–8. [PubMed] [Google Scholar]
- 139.Kirkin V, Cahuzac N, Guardiola-Serrano F, Huault S, Lückerath K, Friedmann E, et al. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 2007;14:1678–87. doi: 10.1038/sj.cdd.4402175. [DOI] [PubMed] [Google Scholar]
- 140.Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, et al. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14:1040–9. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- 141.Malleter M, Tauzin S, Bessede A, Castellano R, Goubard A, Godey F, et al. CD95L cell surface cleavage triggers a prometastatic signaling pathway in triple-negative breast cancer. Cancer Res. 2013;73:6711–21. doi: 10.1158/0008-5472.CAN-13-1794. [DOI] [PubMed] [Google Scholar]
- 142.Poissonnier A, Sanséau D, Le Gallo M, Malleter M, Levoin N, Viel R, et al. CD95-mediated calcium signaling promotes T helper 17 trafficking to inflamed organs in lupus-prone mice. Immunity. 2016;45:209–23. doi: 10.1016/j.immuni.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble fas ligand blocks the killing. J Exp Med. 1997;186:2045–50. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schneider P, Holler N, Bodmer J-L, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tauzin S, Chaigne-Delalande B, Selva E, Khadra N, Daburon S, Contin-Bordes C, et al. The Naturally Processed CD95L Elicits a c-Yes/Calcium/PI3K-Driven Cell Migration Pathway. PLoS Biol. 2011;9:e1001090. doi: 10.1371/journal.pbio.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Le Gallo M, Poissonnier A, Blanco P, Legembre P. CD95/Fas, non-apoptotic signaling pathways, and kinases. Front Immunol. 2017;8. 10.3389/FIMMU.2017.01216. [DOI] [PMC free article] [PubMed]
- 147.O’ Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–63. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ehrenschwender M, Siegmund D, Wicovsky A, Kracht M, Dittrich-Breiholz O, Spindler V, et al. Mutant PIK3CA licenses TRAIL and CD95L to induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death Differ. 2010;17:1435–47. doi: 10.1038/cdd.2010.36. [DOI] [PubMed] [Google Scholar]
- 149.Cursi S, Rufini A, Stagni V, Condò I, Matafora V, Bachi A, et al. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J. 2006;25:1895–905. doi: 10.1038/sj.emboj.7601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Powley IR, Hughes MA, Cain K, MaCfarlane M. Caspase-8 tyrosine-380 phosphorylation inhibits CD95 DISC function by preventing procaspase-8 maturation and cycling within the complex. Oncogene. 2016;35:5629–40. doi: 10.1038/onc.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 2007;67:11505–9. doi: 10.1158/0008-5472.CAN-07-5755. [DOI] [PubMed] [Google Scholar]
- 152.Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–48. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 153.Drachsler M, Kleber S, Mateos A, Volk K, Mohr N, Chen S, et al. CD95 maintains stem cell-like and non-classical EMT programs in primary human glioblastoma cells. Cell Death Dis. 2016;7. 10.1038/CDDIS.2016.102. [DOI] [PMC free article] [PubMed]
- 154.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–95. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 155.Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–9. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 156.Xing Y, Hogquist KA. T-Cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012;4:1–15. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gururajan M, Sindhava VJ, Bondada S. B cell tolerance in health and disease. Antibodies. 2014;3:116–29. [Google Scholar]
- 158.Opferman JT. Apoptosis in the development of the immune system. Cell Death Differ. 2007;15:234–42. doi: 10.1038/sj.cdd.4402182. [DOI] [PubMed] [Google Scholar]
- 159.Strasser A, Puthalakath H, O’Reilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 160.Siegel RM, Chan FKM, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–74. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 161.Green DR, Ferguson TA. The role of fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–24. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 162.Brunner T, Wasem C, Torgler R, Cima I, Jakob S, Corazza N. Fas (CD95/Apo-1) ligand regulation in T cell homeostasis, cell-mediated cytotoxicity and immune pathology. Semin Immunol. 2003;15:167–76. doi: 10.1016/s1044-5323(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 163.Yamada A, Arakaki R, Saito M, Kudo Y, Ishimaru N. Dual role of Fas/FasL-mediated signal in peripheral immune tolerance. Front Immunol. 2017;8:403. doi: 10.3389/fimmu.2017.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–9. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 165.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 166.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, et al. Fas ligand mediates activation-induced cell death in human t lymphocytes. J Exp Med. 1995;181:71–7. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornand T, et al. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–4. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 168.Mariani SM, Krammer PH. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973–82. doi: 10.1002/(SICI)1521-4141(199803)28:03<973::AID-IMMU973>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 169.Tinhofer I, Marschitz I, Kos M, Henn T, Egle A, Villunger A, et al. Differential sensitivity of CD4+ and CD8+T lymphocytes to the killing efficacy of Fas (Apo-1/CD95) Ligand+ tumor cells in B chronic lymphocytic leukemia. Blood. 1998;91:4273–81. [PubMed] [Google Scholar]
- 170.Hsu AP, Dowdell KC, Davis J, Niemela JE, Anderson SM, Shaw PA, et al. Autoimmune lymphoproliferative syndrome due to FAS mutations outside the signal-transducing death domain: molecular mechanisms and clinical penetrance. Genet Med. 2012;14:81–9. doi: 10.1038/gim.0b013e3182310b7d. [DOI] [PubMed] [Google Scholar]
- 171.Paulsen M, Janssen O. Pro- and anti-apoptotic CD95 signaling in T cells. Cell Commun Signal. 2011;9:7. doi: 10.1186/1478-811X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Holzelova E, Vonarbourg C, Stolzenberg M-C, Arkwright PD, Selz F, Prieur A-M, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351:1409–18. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 173.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, et al. Mutations in fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 174.Del-Rey M, Ruiz-Contreras J, Bosque A, Calleja S, Gomez-Rial J, Roldan E, et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood. 2006;108:1306–12. doi: 10.1182/blood-2006-04-015776. [DOI] [PubMed] [Google Scholar]
- 175.Miano M, Cappelli E, Pezzulla A, Venè R, Grossi A, Terranova P, et al. FAS-mediated apoptosis impairment in patients with ALPS/ALPS-like phenotype carrying variants on CASP10 gene. Br J Haematol. 2019;187:502–8. doi: 10.1111/bjh.16098. [DOI] [PubMed] [Google Scholar]
- 176.Seyrek K, Ivanisenko NV, Wohlfromm F, Espe J, Lavrik IN. Impact of human CD95 mutations on cell death and autoimmunity: a model. Trends Immunol. 2021. 10.1016/J.IT.2021.11.006. [DOI] [PubMed]
- 177.Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rösen-Wolff A, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 178.Turbyville JC, Rao VK. The autoimmune lymphoproliferative syndrome: A rare disorder providing clues about normal tolerance. Autoimmun Rev. 2010;9:488–93. doi: 10.1016/j.autrev.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 179.Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989–99. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118:5741. doi: 10.1182/blood-2011-07-325217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153:1541–50. doi: 10.1016/S0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Molnár E, Radwan N, Kovács G, Andrikovics H, Henriquez F, Zarafov A, et al. Key diagnostic markers for autoimmune lymphoproliferative syndrome with molecular genetic diagnosis. Blood. 2020;136:1933–45. doi: 10.1182/blood.2020005486. [DOI] [PubMed] [Google Scholar]
- 183.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. [DOI] [PMC free article] [PubMed]
- 184.Caminha I, Fleisher TA, Hornung RL, Dale JK, Niemela JE, Price S, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2010;125:946. doi: 10.1016/j.jaci.2009.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, et al. FAS-L, IL-10, and double-negative CD4 -CD8 - TCR α/β + T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–30. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 186.Wallach-Dayan SB, Petukhov D, Ahdut-Hacohen R, Richter-Dayan M, Breuer R. Sfasl—the key to a riddle: Immune responses in aging lung and disease. Int J Mol Sci. 2021;22:1–12. doi: 10.3390/ijms22042177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim Biophys Acta. 2017;1864:2015–25. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 188.Cohen PL, Eisenberg RA. The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today. 1992;13:427–8. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- 189.Schile A, Petrillo M, Vovk A, French R, Leighton K, Dragos Z, et al. A comprehensive phenotyping program for the MRL-lpr mouse lupus model. J Immunol. 2018;200(1 Supplement) 40.2.
- 190.Watanabe T, Sakai Y, Miyawaki S, Shimizu A, Koiwai O, Ohno K. A molecular genetic linkage map of mouse chromosome 19, including the lpr, Ly-44, and Tdt genes. Biochemical Genet. 1991;29:325–35. doi: 10.1007/BF00554140. [DOI] [PubMed] [Google Scholar]
- 191.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 192.Perry D, Sang A, Yin Y, Zheng Y-Y, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011. 10.1155/2011/271694. [DOI] [PMC free article] [PubMed]
- 193.Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol. 2017;29:434–41. doi: 10.1097/BOR.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–82. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 196.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yoshida H, Satoh M, Behney KM, Lee CG, Richards HB, Shaheen VM, et al. Effect of an exogenous trigger on the pathogenesis of lupus in (NZB x NZW)F1 mice. Arthritis Rheum. 2002;46:2235–44. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Appleby P, Webber DG, Bowen JG. Murine chronic graft-versus-host disease as a model of systemic lupus erythematosus: effect of immunosuppressive drugs on disease development. Clin Exp Immunol. 1989;78:449. [PMC free article] [PubMed] [Google Scholar]
- 199.Richard ML, Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 2018;5:199. doi: 10.1136/lupus-2016-000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Reid S, Alexsson A, Frodlund M, Morris D, Sandling JK, Bolin K, et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann Rheum Dis. 2019. 10.1136/annrheumdis-2019-216227. [DOI] [PMC free article] [PubMed]
- 201.Neven B, Magerus-Chatinet A, Florkin B, Gobert D, Lambotte O, De Somer L, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118:4798–807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 202.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Investig. 1996;98:1107–13. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bashal F. Hematological disorders in patients with systemic lupus erythematosus. Open Rheumatol J. 2013;7:87. doi: 10.2174/1874312901307010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci. 2013;346:319–23. doi: 10.1097/MAJ.0b013e31827f4ee3. [DOI] [PubMed] [Google Scholar]
- 205.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–9. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 206.Chatham WW, Kimberly RP. Treatment of lupus with corticosteroids. Lupus. 2001;10:140–7. [DOI] [PubMed]
- 207.Lim CC, Liu PY, Tan HZ, Lee P, Chin YM, Mok IY, et al. Severe infections in patients with lupus nephritis treated with immunosuppressants: a retrospective cohort study. Nephrology. 2017;22:478–84. doi: 10.1111/nep.12809. [DOI] [PubMed] [Google Scholar]
- 208.Gu C, Zhao R, Zhang X, Gu Z, Zhou W, Wang Y, et al. A meta-analysis of secondary osteoporosis in systemic lupus erythematosus: prevalence and risk factors. Arch Osteoporos. 2019;15:1–12. doi: 10.1007/s11657-019-0667-1. [DOI] [PubMed] [Google Scholar]
- 209.Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs. 2018;78:355–66. doi: 10.1007/s40265-018-0872-z. [DOI] [PubMed] [Google Scholar]
- 210.D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, et al. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–70. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Božič B, Rozman B. Apoptosis and autoimmunity. EJIFCC. 2006;17:69–74. [PMC free article] [PubMed] [Google Scholar]
- 212.Ishimaru N, Yanagi K, Ogawa K, Suda T, Saito I, Hayashi Y. Possible role of organ-specific autoantigen for Fas ligand-mediated activation-induced cell death in murine sjögren’s syndrome. J Immunol. 2001;167:6031–7. doi: 10.4049/jimmunol.167.10.6031. [DOI] [PubMed] [Google Scholar]
- 213.Trivedi PM, Fynch S, Kennedy LM, Chee J, Krishnamurthy B, O’Reilly LA, et al. Soluble FAS ligand is not required for pancreatic islet inflammation or beta-cell destruction in non-obese diabetic mice. Cell Death Discov. 2019;5. 10.1038/s41420-019-0217-z. [DOI] [PMC free article] [PubMed]
- 214.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Trauth BC, Klas C, Peters AMJ, Matzku S, Möller P, Falk W, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–5. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 216.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Sameshima M, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 217.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 218.Kakinuma C, Takagaki K, Yatomi T, Nakamura N, Nagata S, Uemura A, et al. Acute toxicity of an anti-Fas antibody in mice. Toxicol Pathol. 1999;27:412–20. doi: 10.1177/019262339902700404. [DOI] [PubMed] [Google Scholar]
- 219.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 220.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles in the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–13. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 221.Richards DM, Merz C, Gieffers C, Krendyukov A. CD95L and anti-tumor immune response: current understanding and new evidence. Cancer Manag Res. 2021;13:2477–82. doi: 10.2147/CMAR.S297499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kurooka M, Nuovo GJ, Caligiuri MA, Nabel GJ. Cellular localization and function of Fas ligand (CD95L) in tumors. Cancer Res. 2002;62:1261–5. [PubMed] [Google Scholar]
- 223.Mitsiades N, Poulaki V, Mastorakos G, Tseleni-Balafouta S, Kotoula V, Koutras DA, et al. Fas ligand expression in thyroid carcinomas: a potential mechanism of immune evasion. J Clin Endocrinol Metab. 1999;84:2924–32. doi: 10.1210/jcem.84.8.5917. [DOI] [PubMed] [Google Scholar]
- 224.Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4. [PubMed] [Google Scholar]
- 225.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: Implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 226.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonie adenocarcinomas. Proc Natl Acad Sci USA. 1997;94:6420–5. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gastman BR, Atarashi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, et al. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–64. [PubMed] [Google Scholar]
- 228.Moers C, Warskulat U, Even J, Niederacher D, Beckmann MW, Müschen M. CD95 ligand expression as a mechanism of immune escape in breast cancer. Immunology. 2000;99:69–77. doi: 10.1046/j.1365-2567.2000.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–15. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhu J, Powis De Tenbossche CG, Cané S, Colau D, Van Baren N, Lurquin C, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun. 2017;8:1–15. doi: 10.1038/s41467-017-00784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–73. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 233.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+T lymphocytes. J Immunol. 2009;183:3720–30. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Taghikhani A, Farzaneh F, Sharifzad F, Mardpour S, Ebrahimi M, Hassan ZM. Engineered tumor-derived extracellular vesicles: potentials in cancer immunotherapy. Front Immunol. 2020;11. 10.3389/fimmu.2020.00221. [DOI] [PMC free article] [PubMed]
- 235.De La Motte Rouge T, Corné J, Cauchois A, Le Boulch M, Poupon C, Henno S, et al. Serum CD95L level correlates with tumor immune infiltration and is a positive prognostic marker for advanced high-grade serous ovarian cancer. Mol Cancer Res. 2019;17:2537–48. doi: 10.1158/1541-7786.MCR-19-0449. [DOI] [PubMed] [Google Scholar]
- 236.Wojtukiewicz MZ, Rek MM, Karpowicz K, Górska M, Polityńska B, Wojtukiewicz AM, et al. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40:949–82. doi: 10.1007/s10555-021-09976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:1–12. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hahn AW, Gill DM, Pal SK, Agarwal N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy. 2017;9:681–92. doi: 10.2217/imt-2017-0024. [DOI] [PubMed] [Google Scholar]
- 239.Burton EM, Tawbi HA. Bispecific antibodies to PD-1 and CTLA4: doubling down on T cells to decouple efficacy from toxicity. Cancer Discov. 2021;11:1008–10. doi: 10.1158/2159-8290.CD-21-0257. [DOI] [PubMed] [Google Scholar]
- 240.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2018;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chavez ARDV, Buchser W, Basse PH, Liang X, Appleman LJ, Maranchie JK, et al. Pharmacologic administration of interleukin-2. Ann NY Acad Sci. 2009;1182:14–27. doi: 10.1111/j.1749-6632.2009.05160.x. [DOI] [PubMed] [Google Scholar]
- 242.Skrombolas D, Frelinger JG. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev Clin Immunol. 2014;10:207–17. doi: 10.1586/1744666X.2014.875856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xue D, Hsu E, Fu Y-X, Peng H. Next-generation cytokines for cancer immunotherapy. Antib Therapeutics. 2021;4:123–33. doi: 10.1093/abt/tbab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, et al. Human lung carcinomas express fas ligand. Cancer Res. 1997;57:1007–12. [PubMed] [Google Scholar]
- 246.Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, et al. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Investig. 1997;99:1173–8. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, Ohgaki H. Up-regulation of Fas (APO-1/CD95) ligand and down-regulation of Fas expression in human esophageal cancer. Cancer Res. 1998;58:2057–62. [PubMed] [Google Scholar]
- 248.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, et al. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;58:1741–9. [PubMed] [Google Scholar]
- 249.Liu Q-Y, Rubin MA, Omene C, Lederman S, Stein CA. Fas ligand is constitutively secreted by prostate cancer cells in vitro. Clin Cancer Res. 1998;4:1803–11. [PubMed] [Google Scholar]
- 250.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 251.Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–45. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Friesen C, Fulda S, Debatin KM. Cytotoxic drugs and the CD95 pathway. Leukemia. 1999;13:1854–8. doi: 10.1038/sj.leu.2401333. [DOI] [PubMed] [Google Scholar]
- 253.Mo YY, Beck WT. DNA damage signals induction of Fas ligand in tumor cells. Mol Pharmacol. 1999;55:216–22. doi: 10.1124/mol.55.2.216. [DOI] [PubMed] [Google Scholar]
- 254.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 255.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–805. [PubMed] [Google Scholar]
- 256.McSweeney KM, Bozza WP, Alterovitz W-L, Zhang B. Transcriptomic profiling reveals p53 as a key regulator of doxorubicin-induced cardiotoxicity. Cell Death Discov. 2019;5:1–11. doi: 10.1038/s41420-019-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Li J, Wang P, Long NA, Zhuang J, Springer DA, Zou J, et al. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc Natl Acad Sci. 2019;116:19626–34. doi: 10.1073/pnas.1904979116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Van Dalen E, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochr Database Syst Rev. 2008. 10.1002/14651858.CD003917.pub3. [DOI] [PubMed]
- 259.Sacco G, Bigioni M, Evangelista S, Goso C, Manzini S, Maggi CA. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol. 2001;414:71–8. doi: 10.1016/s0014-2999(01)00782-8. [DOI] [PubMed] [Google Scholar]
- 260.van Acker FA, Boven E, Kramer K, Haenen GR, Bast A, van der Vijgh WJ. Frederine, a new and promising protector against doxorubicin-induced cardiotoxicity. Clin Cancer Res. 2001;7:1378–84. [PubMed] [Google Scholar]
- 261.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–62. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 262.Todorova VK, Siegel ER, Kaufmann Y, Kumarapeli A, Owen A, Wei JY, et al. Dantrolene attenuates cardiotoxicity of doxorubicin without reducing its antitumor efficacy in a breast cancer model. Transl Oncol. 2020;13:471–80. doi: 10.1016/j.tranon.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu S, Li R, Qian J, Sun J, Li G, Shen J, et al. Combination therapy of doxorubicin and quercetin on multidrug-resistant breast cancer and their sequential delivery by reduction-sensitive hyaluronic acid-based conjugate/d-α-Tocopheryl Poly(ethylene glycol) 1000 Succinate Mixed Micelles. Mol Pharm. 2020;17:1415–27. doi: 10.1021/acs.molpharmaceut.0c00138. [DOI] [PubMed] [Google Scholar]
- 264.DeMaria PJ, Bilusic M. Cancer vaccines. Hematol Oncol Clin North Am. 2001;15:741. doi: 10.1016/j.hoc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 265.Orbach A, Rachmilewitz J, Parnas M, Huang J-H, Tykocinski ML, Dranitzki-Elhalel M. CTLA-4. FasL induces early apoptosis of activated T cells by interfering with anti-apoptotic signals. J Immunol. 2007;179:7287–94. doi: 10.4049/jimmunol.179.11.7287. [DOI] [PubMed] [Google Scholar]
- 266.Orbach A, Rachmilewitz J, Shani N, Isenberg Y, Parnas M, Huang JH, et al. CD40·FasL and CTLA-4·FasL fusion proteins induce apoptosis in malignant cell lines by dual signaling. Am J Pathol. 2010;177:3159–68. doi: 10.2353/ajpath.2010.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Huang JH, Tykocinski ML. CTLA-4-Fas ligand functions as a trans signal converter protein in bridging antigen-presenting cells and T cells. Int Immunol. 2001;13:529–39. doi: 10.1093/intimm/13.4.529. [DOI] [PubMed] [Google Scholar]
- 268.Ho MY, Sun GH, Leu SJJ, Ka SM, Tang SJ, Sun KH. Combination of Fasl and GM-CSF confers synergistic antitumor immunity in an in vivo model of the murine Lewis lung carcinoma. Int J Cancer. 2008;123:123–33. doi: 10.1002/ijc.23474. [DOI] [PubMed] [Google Scholar]
- 269.Bajic D, Chester K, Neri D. An antibody-tumor necrosis factor fusion protein that synergizes with oxaliplatin for treatment of colorectal cancer. Mol Cancer Ther. 2020;19:2554–63. doi: 10.1158/1535-7163.MCT-19-0729. [DOI] [PubMed] [Google Scholar]
- 270.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–42. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 271.Jung G, Grosse-Hovest L, Krammer PH, Rammensee HG. Target cell-restricted triggering of the CD95 (APO-1/Fas) death receptor with bispecific antibody fragments. Cancer Res. 2001;61:1846–8. [PubMed] [Google Scholar]
- 272.Herrmann T, Große-Hovest L, Otz T, Krammer PH, Rammensee HG, Jung G. Construction of optimized bispecific antibodies for selective activation of the death receptor CD95. Cancer Res. 2008;68:1221–7. doi: 10.1158/0008-5472.CAN-07-6175. [DOI] [PubMed] [Google Scholar]
- 273.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Busch DH, Fräßle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28:28. doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sankhla SK, Nadkarni JS, Bhagwati SN. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J Neuro Oncol. 1996;27:133–40. doi: 10.1007/BF00177476. [DOI] [PubMed] [Google Scholar]
- 276.Kumar A, Watkins R, Vilgelm AE. Cell therapy with TILs: training and taming T cells to fight cancer. Front Immunol. 2021;12. 10.3389/FIMMU.2021.690499. [DOI] [PMC free article] [PubMed]
- 277.Chacon JA, Pilon-Thomas S, Sarnaik AA, Radvanyi LG. Continuous 4-1BB co-stimulatory signals for the optimal expansion of tumor-infiltrating lymphocytes for adoptive T-cell therapy. Oncoimmunology. 2013;2. 10.4161/ONCI.25581. [DOI] [PMC free article] [PubMed]
- 278.Chacon JA, Sarnaik AA, Pilon-Thomas S, Radvanyi L. Triggering co-stimulation directly in melanoma tumor fragments drives CD8+ tumor-infiltrating lymphocyte expansion with improved effector-memory properties. Oncoimmunology. 2015;4.10.1080/2162402X.2015.1040219. [DOI] [PMC free article] [PubMed]
- 279.Tschumi BO, Dumauthioz N, Marti B, Zhang L, Schneider P, Mach JP, et al. CART cells are prone to Fas- and DR5-mediated cell death. J Immunother Cancer. 2018;6:1–9. doi: 10.1186/s40425-018-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wagner SC, Ichim TE, Bogin V, Min WP, Silva F, Patel AN, et al. Induction and characterization of anti-tumor endothelium immunity elicited by ValloVax therapeutic cancer vaccine. Oncotarget. 2017;8:28595–613. doi: 10.18632/oncotarget.15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yamamoto TN, Lee PH, Vodnala SK, Gurusamy D, Kishton RJ, Yu Z, et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J Clin Investig. 2019;129:1551–65. doi: 10.1172/JCI121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Upadhyay R, Boiarsky JA, Pantsulaia G, Svensson-Arvelund J, Lin MJ, Wroblewska A, et al. A critical role for fas-mediated off-target tumor killing in t-cell immunotherapy. Cancer Discov. 2021;11:599–613. doi: 10.1158/2159-8290.CD-20-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tritz R, Hickey MJ, Lin AH, Hadwiger P, Sah DWY, Neuwelt EA, et al. FAPP2 gene downregulation increases tumor cell sensitivity to Fas-induced apoptosis. Biochem Biophys Res Commun. 2009;383:167–71. doi: 10.1016/j.bbrc.2009.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tritz R, Mueller BM, Hickey MJ, Lin AH, Gomez GG, Hadwiger P, et al. siRNA down-regulation of the PATZ1 gene in human glioma cells increases their sensitivity to apoptotic stimuli. Cancer Ther. 2008;6:865–76. [PMC free article] [PubMed] [Google Scholar]
- 285.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–73. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 286.Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163–77.e21. doi: 10.1016/j.cell.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wei Y, Li J, Kotturi HSR. Cancer gene therapy via NKG2D and FAS pathways. Targets Gene Ther. 2011. 10.5772/17386.
- 288.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–43. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 289.Jalil AR, Andrechak JC, Discher DE. Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPα structure–function. Antib Ther. 2020;3:80. doi: 10.1093/abt/tbaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Aronin A, Amsili S, Prigozhina TB, Tzdaka K, Shen R, Grinmann L, et al. Highly efficient, In-vivo Fas-mediated apoptosis of B-cell lymphoma by hexameric CTLA4-FasL. J Hematol Oncol. 2014;7:1–15. doi: 10.1186/s13045-014-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Aronin A, Ben Gigi-Tamir L, Makdasi E, Amsili S, Ben David S, Avraham O, et al. CTLA4-FasL, the hexameric targeted FasL fusion protein, as potential novel treatment for DLBCL. Blood. 2017;130:4107. [Google Scholar]
- 292.Daburon S, Devaud C, Costet P, Morello A, Garrigue-Antar L, Maillasson M, et al. Functional characterization of a chimeric soluble fas ligand polymer with in vivo anti-tumor activity. PLoS ONE. 2013;8:54000. doi: 10.1371/journal.pone.0054000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Taupin JL, Miossec V, Pitard V, Blanchard F, Daburon S, Raher S, et al. Binding of leukemia inhibitory factor (LIF) to mutants of its low affinity receptor, gp190, reveals a LIF binding site outside and interactions between the two cytokine binding domains. J Biol Chem. 1999;274:14482–9. doi: 10.1074/jbc.274.20.14482. [DOI] [PubMed] [Google Scholar]
- 294.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–40. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Monet M, Poët M, Tauzin S, Fouqué A, Cophignon A, Lagadic-Gossmann D, et al. The cleaved FAS ligand activates the Na+/H+ exchanger NHE1 through Akt/ROCK1 to stimulate cell motility. Sci Rep. 2016;6:1–9. doi: 10.1038/srep28008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Edmond V, Dufour F, Poiroux G, Shoji K, Malleter M, Fouqué A, et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene. 2014;34:996–1005. doi: 10.1038/onc.2014.55. [DOI] [PubMed] [Google Scholar]
- 297.Gieffers C, Kunz C, Sykora J, Merz C, Thiemann M, Fricke H, et al. Abstract 464: Methylation of a single CpG site in the CD95-ligand promoter is a biomarker predicting the response to therapy with APG101 in glioblastoma. Cancer Res. 2016;76:464. [Google Scholar]
- 298.Farrukh, Zhou Y, Jin L, Xu Y, Zhang J, Chen H, et al. CpG2 hypermethylation in the CD95L promoter is associated with survival in patients with glioblastoma: an observational study. Glioma. 2021;4:22. [Google Scholar]
- 299.Teng Y, Dong Y, Liu Z, Zou Y, Xie H, Zhao Y, et al. DNA methylation-mediated caspase-8 downregulation is associated with anti-apoptotic activity and human malignant glioma grade. Int J Mol Med. 2017;39:725–33. doi: 10.3892/ijmm.2017.2881. [DOI] [PubMed] [Google Scholar]
- 300.Gopisetty G, Ramachandran K, Singal R. DNA methylation and apoptosis. Mol Immunol. 2006;43:1729–40. doi: 10.1016/j.molimm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 301.Lu C, Klement JD, Yang D, Albers T, Lebedyeva IO, Waller JL, et al. SUV39H1 regulates human colon carcinoma apoptosis and cell cycle to promote tumor growth. Cancer Lett. 2020;476:87. doi: 10.1016/j.canlet.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730–8. doi: 10.1038/nm.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dhib-Jalbut S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology. 2007;68. 10.1212/01.WNL.0000275228.13012.7B. [DOI] [PubMed]
- 304.Ömerhoca S, Akkaş SY, İçen NK. Multiple sclerosis: diagnosis and differential diagnosis. Arch Neuropsychiatry. 2018;55:S1. doi: 10.29399/npa.23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dastagir A, Healy BC, Chua AS, Chitnis T, Weiner HL, Bakshi R, et al. Brain and spinal cord MRI lesions in primary progressive vs. relapsing-remitting multiple sclerosis. eNeurologicalSci. 2018;12:42. doi: 10.1016/j.ensci.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tegla CA, Azimzadeh P, Andrian-Albescu M, Martin A, Cudrici CD, Trippe R, et al. SIRT1 is decreased during relapses in patients with multiple sclerosis. Exp Mol Pathol. 2014;96:139–48. doi: 10.1016/j.yexmp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 307.Kruszewski AM, Rao G, Tatomir A, Hewes D, Tegla CA, Cudrici CD, et al. RGC-32 as a potential biomarker of relapse and response to treatment with glatiramer acetate in multiple sclerosis. Exp Mol Pathol. 2015;99:498–505. doi: 10.1016/j.yexmp.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tatomir A, Talpos-Caia A, Anselmo F, Kruszewski AM, Boodhoo D, Rus V, et al. The complement system as a biomarker of disease activity and response to treatment in multiple sclerosis. Immunol Res. 2017;65:1103. doi: 10.1007/s12026-017-8961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Hewes D, Tatomir A, Kruszewski AM, Rao G, Tegla CA, Ciriello J, et al. SIRT1 as a potential biomarker of response to treatment with glatiramer acetate in multiple sclerosis. Exp Mol Pathol. 2017;102:191–7. doi: 10.1016/j.yexmp.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 310.Zhong W, Jiang Y, Ma H, Wu J, Jiang Z, Zhao L. Elevated levels of CCR6+ T helper 22 cells correlate with skin and renal impairment in systemic lupus erythematosus. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-13344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kim SJ, Lee K, Diamond B. Follicular helper T cells in systemic lupus erythematosus. Front Immunol. 2018;9:1793. doi: 10.3389/fimmu.2018.01793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yoshitomi H, Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol. 2020;18:523–7. doi: 10.1038/s41423-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gryzik S, Hoang Y, Lischke T, Mohr E, Venzke M, Kadner I, et al. Identification of a super-functional TFH-like subpopulation in murine lupus by pattern perception. eLife. 2020;9. 10.7554/ELIFE.53226. [DOI] [PMC free article] [PubMed]
- 314.Cencioni MT, Santini S, Ruocco G, Borsellino G, De Bardi M, Grasso MG, et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis. 2015;6:e1741. doi: 10.1038/cddis.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 316.Steinman L. A brief history of TH17, the first major revision in the T H1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 317.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 319.Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:217–33. doi: 10.1038/nrrheum.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Taraborrelli L, Peltzer N, Montinaro A, Kupka S, Rieser E, Hartwig T, et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat Commun. 2018;9. 10.1038/S41467-018-06155-8. [DOI] [PMC free article] [PubMed]
- 321.Annibaldi A, Walczak H. Death receptors and their ligands in inflammatory disease and cancer. Cold Spring Harb Perspect Biol. 2020;12:1–19. doi: 10.1101/cshperspect.a036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 323.Hsieh SL, Lin WW. Decoy receptor 3: An endogenous immunomodulator in cancer growth and inflammatory reactions. J Biomed Sci. 2017;24. 10.1186/s12929-017-0347-7. [DOI] [PMC free article] [PubMed]
- 324.Blaes J, Thom CM, Pfenning P-N, Ubmann PR, Sahm F, Wick A, et al. Cell death and survival inhibition of CD95/CD95L (FAS/FASLG) signaling with APG101 prevents invasion and enhances radiation therapy for glioblastoma. Cancer Res. 2018;16:767–76. 10.1158/1541-7786.MCR-17-0563. [DOI] [PubMed]
- 325.Merz C, Strecker A, Sykora J, Hill O, Fricke H, Angel P, et al. Neutralization of the CD95 ligand by APG101 inhibits invasion of glioma cells in vitro. Anticancer drugs. 2015;26:716–27. doi: 10.1097/CAD.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J Immunol. 2011;187:5901–9. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yolcu ES, Gu X, Lacelle C, Zhao H, Bandura-Morgan L, Askenasy N, et al. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol. 2008;181:931–9. doi: 10.4049/jimmunol.181.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Askenasy N, Yolcu ES, Wang Z, Shirwan H. Display of Fas ligand protein on cardiac vasculature as a novel means of regulating allograft rejection. Circulation. 2003;107:1525–31. doi: 10.1161/01.cir.0000064893.96179.7e. [DOI] [PubMed] [Google Scholar]
- 329.Makdasi E, Amsili S, Aronin A, Prigozhina TB, Tzdaka K, Gozlan YM, et al. Toxicology and pharmacokinetic studies in mice and nonhuman primates of the nontoxic, efficient, targeted hexameric FasL: CTLA4-FasL. Mol Cancer Ther. 2020;19:513–24. doi: 10.1158/1535-7163.MCT-19-0558. [DOI] [PubMed] [Google Scholar]
- 330.Vacaru A-M, Dumitrescu M, Vacaru AM, Fenyo IM, Ionita R, Gafencu AV, et al. Enhanced suppression of immune cells in vitro by MSC overexpressing FasL. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal stem cell-induced immunoregulation involves Fas ligand/Fas-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 333.Chen C, Akiyama K, Yamaza T, You Y-O, Xu X, Li B, et al. Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med. 2014;6:322–34. doi: 10.1002/emmm.201303000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhong H, Wang H, Li J, Huang Y. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol Sin. 2019;40:1373–85. doi: 10.1038/s41401-019-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ralff MD, El-Deiry WS. TRAIL pathway targeting therapeutics. Expert Rev Precis Med Drug Dev. 2018;3:197. doi: 10.1080/23808993.2018.1476062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Gerspach J, Pfizenmaier K, Wajant H. Therapeutic targeting of CD95 and the TRAIL death receptors. Recent Pat Anticancer Drug Discov. 2011;6:294–310. doi: 10.2174/157489211796957739. [DOI] [PubMed] [Google Scholar]
- 337.Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-α therapies: the next generation. Nat Rev Drug Discov. 2003;2:736–46. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 338.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27:55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Gerriets V, Goyal A, Khaddour K. Tumor Necrosis Factor Inhibitors. [Updated 2021 Jul 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482425/. [PubMed]
- 340.Mocci G, Marzo M, Papa A, Armuzzi A, Guidi L. Dermatological adverse reactions during anti-TNF treatments: focus on inflammatory bowel disease. J Crohn’s Colitis. 2013;7:769–79. doi: 10.1016/j.crohns.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 341.Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA. Neurological adverse events in patients receiving anti-TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res Ther. 2014;16. 10.1186/AR4582. [DOI] [PMC free article] [PubMed]
- 342.Shivaji UN, Sharratt CL, Thomas T, Smith SCL, Iacucci M, Moran GW, et al. Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Alimentary Pharmacol Ther. 2019;49:664–80. doi: 10.1111/apt.15097. [DOI] [PubMed] [Google Scholar]
- 343.Chen X, Nie K, Zhang X, Tan S, Zheng Q, Wang Y, et al. The recombinant anti-TNF-α fusion protein ameliorates rheumatoid arthritis by the protective role of autophagy. Biosci Rep. 2020;40:20194515. doi: 10.1042/BSR20194515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liesche C, Berndt J, Fricke F, Aschenbrenner S, Heilemann M, Eils R, et al. CD95 receptor activation by ligand-induced trimerization is independent of its partial pre-ligand assembly Insights into CD95 receptor activation by quantitative fluorescence microscopy approaches. bioRxiv 293530; 10.1101/293530.
- 345.Pfizenmaier K, Wajant H. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced. J Immunol. 2009. 10.4049/jimmunol.0802597. [DOI] [PubMed]
- 346.Krendyukov A, Gieffers C. Asunercept as an innovative therapeutic approach for recurrent glioblastoma and other malignancies. Cancer Manag Res. 2019;11:8095. doi: 10.2147/CMAR.S216675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, et al. A phase II, randomized, study of weekly APG101+ reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20:6304–13. doi: 10.1158/1078-0432.CCR-14-0951-T. [DOI] [PubMed] [Google Scholar]
- 348.Raimbault A, Pierre-Eugene C, Rouquette A, Deudon C, Willems L, Chapuis N, et al. APG101 efficiently rescues erythropoiesis in lower risk myelodysplastic syndromes with severe impairment of hematopoiesis. Oncotarget. 2016;7:14898–911. doi: 10.18632/oncotarget.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Boch T, Luft T, Metzgeroth G, Mossner M, Jann JC, Nowak D, et al. Safety and efficacy of the CD95-ligand inhibitor asunercept in transfusion-dependent patients with low and intermediate risk MDS. Leuk Res. 2018;68:62–9. doi: 10.1016/j.leukres.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 350.Obungu VH, Gelfanova V, Rathnachalam R, Bailey A, Sloan-Lancaster J, Huang L. Determination of the mechanism of action of Anti-FasL antibody by epitope mapping and homology modeling. Biochemistry. 2009;48:7251–60. doi: 10.1021/bi900296g. [DOI] [PubMed] [Google Scholar]
- 351.Nalivaiko K, Hofmann M, Kober K, Teichweyde N, Krammer PH, Rammensee HG, et al. A Recombinant bispecific CD20×CD95 antibody with superior activity against normal and malignant B-cells. Mol Ther. 2016;24:298–305. doi: 10.1038/mt.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med. 2012;18:529–37. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- 353.Longley DB, Allen WL, McDermott U, Wilson TR, Latif T, Boyer J, et al. The roles of thymidylate synthase and p53 in regulating Fas-mediated apoptosis in response to antimetabolites. Clin Cancer Res. 2004;10:3562–71. doi: 10.1158/1078-0432.CCR-03-0532. [DOI] [PubMed] [Google Scholar]
- 354.McDermott U, Galligan L, Longley DB, Scullin P, Johnston PG. Fas-mediated apoptosis in response to Irinotecan (CPT-11) is p53-independent and STAT1-dependent. Cancer Res. 2005;65(9_Supplement):1266.5.
- 355.Backus HHJ, Dukers DF, Van Groeningen CJ, Vos W, Bloemena E, Wouters D, et al. 5-Fluorouracil induced Fas upregulation associated with apoptosis in liver metastases of colorectal cancer patients. Ann Oncol J Eur Soc Med Oncol. 2001;12:209–16. doi: 10.1023/a:1008331525368. [DOI] [PubMed] [Google Scholar]
- 356.Puviani M, Marconi A, Pincelli C, Cozzani E. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J Investig Dermatol. 2003;120:164–7. doi: 10.1046/j.1523-1747.2003.12014.x. [DOI] [PubMed] [Google Scholar]
- 357.Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, et al. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:679–86. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- 358.Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, et al. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell. 2009;5:178–90. doi: 10.1016/j.stem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 359.Letellier E, Kumar S, Sancho-Martinez I, Krauth S, Funke-Kaiser A, Laudenklos S, et al. CD95-ligand on peripheral myeloid cells activates Syk kinase to trigger their recruitment to the inflammatory site. Immunity. 2010;32:240–52. doi: 10.1016/j.immuni.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 360.Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10:389–96. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- 361.Omokaro SO, Desierto MJ, Eckhaus MA, Ellison FM, Chen J, Young NS. Lymphocytes with aberrant expression of Fas or Fas-ligand attenuate immune bone marrow failure in a mouse model. J Immunol. 2009;182:3414. doi: 10.4049/jimmunol.0801430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Fadeel B, Thorpe CJ, Yonehara S, Chiodi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201–9. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- 363.Teodorczyk M, Kleber S, Wollny D, Sefrin JP, Aykut B, Mateos A, et al. CD95 promotes metastatic spread via Sck in pancreatic ductal adenocarcinoma. Cell Death Differ. 2015;22:1192–202. doi: 10.1038/cdd.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–11. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Atsuta I, Liu S, Miura Y, Akiyama K, Chen C, An Y, et al. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res Ther. 2013;4:111. doi: 10.1186/scrt322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, et al. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21:611–21. doi: 10.1158/1078-0432.CCR-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Chacon JA, Sarnaik AA, Pilon-Thomas S, Radvanyi L. Triggering co-stimulation directly in melanoma tumor fragments drives CD8+ tumor-infiltrating lymphocyte expansion with improved effector-memory properties. Oncoimmunology. 2015;4. 10.1080/2162402X.2015.1040219. [DOI] [PMC free article] [PubMed]
- 368.Nojima T, Haniuda K, Moutai T, Matsudaira M, Mizokawa S, Shiratori I, et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat Commun. 2011;2:1–11. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 369.Gori JL, Hsu PD, Maeder ML, Shen S, Welstead GG, Bumcrot D. Delivery and specificity of CRISPR-Cas9 genome editing technologies for human gene therapy. Hum Gene Ther. 2015;26:443–51. doi: 10.1089/hum.2015.074. [DOI] [PubMed] [Google Scholar]
- 370.Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T-cell subset with stem cell-like properties. Nat Med. 2011;17:1290. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Whartenby KA, Straley EE, Kim H, Racke F, Tanavde V, Gorski KS, et al. Transduction of donor hematopoietic stem-progenitor cells with Fas ligand enhanced short-term engraftment in a murine model of allogeneic bone marrow transplantation. Blood. 2002;100:3147–54. doi: 10.1182/blood-2002-01-0118. [DOI] [PubMed] [Google Scholar]
- 373.Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419–27. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 374.Harding J, Vintersten-Nagy K, Shutova M, Yang H, Tang JK, Massumi M, et al. Induction of long-term allogeneic cell acceptance and formation of immune privileged tissue in immunocompetent hosts. bioRxiv. 2019. 10.1101/716571.
- 375.Ahmetlic F, Fauser J, Riedel T, Bauer V, Flessner C, Hömberg N, et al. Original research: therapy of lymphoma by immune checkpoint inhibitors: the role of T cells, NK cells and cytokine-induced tumor senescence. J Immunother Cancer. 2021;9:1660. doi: 10.1136/jitc-2020-001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kim IK, Park SJ, Park JH, Lee SH, Hong SE, Reed JC, et al. and bromocriptine attenuate cell death mediated by intracellular calcium mobilization. BMB Rep. 2012;45:482–7. doi: 10.5483/BMBRep.2012.45.8.024. [DOI] [PubMed] [Google Scholar]
- 377.Futter CE, Crowston JG, Allan BDS. Interaction with collagen IV protects lens epithelial cells from Fas-dependent apoptosis by stimulating the production of soluble survival factors. Investig Ophthalmol Vis Sci. 2005;46:3256–62. doi: 10.1167/iovs.05-0086. [DOI] [PubMed] [Google Scholar]
- 378.Shih MF, Cherng JY. Protective effects of chlorella-derived peptide against UVC-induced cytotoxicity through inhibition of caspase-3 activity and reduction of the expression of phosphorylated FADD and cleaved PARP-1 in skin fibroblasts. Molecules. 2012;17:9116. doi: 10.3390/molecules17089116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hashimoto H, Tanaka M, Suda T, Tomita T, Hayashida K, Takeuchi E, et al. Soluble Fas ligand in the joints of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1998;41:657–62. doi: 10.1002/1529-0131(199804)41:4<657::AID-ART12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 380.Jeong D, Kim HS, Kim HY, Kang MJ, Jung H, Oh Y, et al. Soluble Fas ligand drives autoantibody-induced arthritis by binding to DR5/TRAIL-R2. Elife. 2021;10. 10.7554/ELIFE.48840. [DOI] [PMC free article] [PubMed]
- 381.Conceição-Silva F, Hahne M, Schröter M, Louis J, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol. 1998;28:237–45. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 382.Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, et al. Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat Mater. 2018;17:732–9. doi: 10.1038/s41563-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Simon AK, Jones E, Richards H, Wright K, Betts G, Godkin A, et al. Regulatory T cells inhibit Fas ligand-induced innate and adaptive tumour immunity. Eur J Immunol. 2007;37:758–67. doi: 10.1002/eji.200636593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Lewis RA, Gross RL, Sall KN, Schiffman RM, Liu CC, Batoosingh AL. The safety and efficacy of bimatoprost/timolol fixed combination: a 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2010;19:424–6. doi: 10.1097/IJG.0b013e3181bdb586. [DOI] [PubMed] [Google Scholar]
- 385.Kural MH, Wang J, Gui L, Yuan Y, Li G, Leiby KL, et al. Fas ligand and nitric oxide combination to control smooth muscle growth while sparing endothelium. Biomaterials. 2019;212:28–38. doi: 10.1016/j.biomaterials.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Viard-Leveugle I, Bullani RR, Meda P, Micheau O, Limat A, Saurat J-H, et al. Intracellular Localization of keratinocyte Fas ligand explains lack of cytolytic activity under physiological conditions. J Biol Chem. 2003;278:16183–8. [DOI] [PubMed]
- 387.Hendriks D, He Y, Koopmans I, Wiersma VR, Ginkel RJ van, Samplonius DF, et al. Programmed death ligand 1 (PD-L1)-targeted TRAIL combines PD-L1-mediated checkpoint inhibition with TRAIL-mediated apoptosis induction. Oncoimmunology. 2016;5. 10.1080/2162402X.2016.1202390. [DOI] [PMC free article] [PubMed]
- 388.Bremer E, Kuijlen J, Samplonius D, Walczak H, De Leij L, Helfrich W. Target cell-restricted and -enhanced apoptosis induction by a scFv:sTRAIL fusion protein with specificity for the pancarcinoma-associated antigen EGP2. Int J Cancer. 2004;109:281–90. doi: 10.1002/ijc.11702. [DOI] [PubMed] [Google Scholar]
- 389.Matute-Bello G, Liles WC, Frevert CW, Dhanireddy S, Ballman K, Wong V, et al. Blockade of the Fas/FasL system improves pneumococcal clearance from the lungs without preventing dissemination of bacteria to the spleen. J Infect Dis. 2005;191:596–606. doi: 10.1086/427261. [DOI] [PubMed] [Google Scholar]
- 390.Wroblewski VJ, Witcher DR, Becker GW, Davis KA, Dou S, Micanovic R, et al. Decoy receptor 3 (DcR3) is proteolytically processed to a metabolic fragment having differential activities against Fas ligand and LIGHT. Biochem Pharmacol. 2003;65:657–67. doi: 10.1016/s0006-2952(02)01612-x. [DOI] [PubMed] [Google Scholar]
- 391.Wroblewski VJ, McCloud C, Davis K, Manetta J, Micanovic R, Witcher DR. Pharmacokinetics, metabolic stability, and subcutaneous bioavailability of a genetically engineered analog of DcR3, FLINT [DcR3(R218Q)], in cynomolgus monkeys and mice. Drug Metab Dispos. 2003;31:502–7. doi: 10.1124/dmd.31.4.502. [DOI] [PubMed] [Google Scholar]
- 392.Einhauer A, Jungbauer A. The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Biophys Methods. 2001;49:455–65. doi: 10.1016/s0165-022x(01)00213-5. [DOI] [PubMed] [Google Scholar]
- 393.Yolcu ES, Gu X, Lacelle C, Zhao H, Bandura-Morgan L, Askenasy N, et al. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol. 2008;181:931–9. doi: 10.4049/jimmunol.181.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J Immunol. 2011;187:5901–9. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–24. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 396.Orbach A, Rachmilewitz J, Parnas M, Huang J-H, Tykocinski ML, Dranitzki-Elhalel M. CTLA-4. FasL induces early apoptosis of activated T cells by interfering with anti-apoptotic signals. J Immunol. 2007;179:7287–94. doi: 10.4049/jimmunol.179.11.7287. [DOI] [PubMed] [Google Scholar]
- 397.Huang J-H, Tykocinski ML. CTLA-4–Fas ligand functions as a trans signal converter protein in bridging antigen-presenting cells and T cells. Int Immunol. 2001;13:529–39. doi: 10.1093/intimm/13.4.529. [DOI] [PubMed] [Google Scholar]
- 398.Ho M, Sun G, Leu SJ, Ka S, Tang S, Sun K. Combination of Fasl and GM‐CSF confers synergistic antitumor immunity in an in vivo model of the murine Lewis lung carcinoma. Int J Cancer. 2008;123:123–33. doi: 10.1002/ijc.23474. [DOI] [PubMed] [Google Scholar]
- 399.Millar DG, Ramjiawan RR, Kawaguchi K, Gupta N, Chen J, Zhang S, et al. Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat Biotechnol. 2020;38:420–5. doi: 10.1038/s41587-019-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Ateh DD, Leinster VH, Lambert SR, Shah A, Khan A, Walklin HJ, et al. The intracellular uptake of CD95 modified paclitaxel-loaded poly(lactic-co-glycolic acid) microparticles. Biomaterials. 2011;32:8538–47. doi: 10.1016/j.biomaterials.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 401.Rieger R, Whitacre D, Cantwell MJ, Prussak C, Kipps TJ. Chimeric form of tumor necrosis factor-α has enhanced surface expression and antitumor activity. Cancer Gene Ther. 2009;16:53–64. doi: 10.1038/cgt.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Tuettenberg J, Seiz M, Debatin KM, Hollburg W, Von Staden M, Thiemann M, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of APG101, a CD95-Fc fusion protein, in healthy volunteers and two glioma patients. Int Immunopharmacol. 2012;13:93–100. doi: 10.1016/j.intimp.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 403.Boldin MP, Goncharov TM, Goltseve YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor–induced cell death. Cell. 1996;85:803–15. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 404.Quinn TP, Soifer SJ, Ramer K, Williams LT, Nakamura MC. A receptor for vascular endothelial growth factor that stimulates endothelial apoptosis. Cancer Res. 2001;61:8629–37. [PubMed] [Google Scholar]
- 405.Quinn T, Niemi E, Nakamura M. Abstract 2176: R1FasL: a recombinant fusion protein that converts VEGF to act as a cell death factor. Cancer Res. 2013;73:2176–2176. [Google Scholar]
- 406.Heinzelmann F, Jendrossek V, Lauber K, Nowak K, Eldh T, Boras R, et al. Irradiation-induced pneumonitis mediated by the CD95/CD95-ligand system. JNCI J Natl Cancer Inst. 2006;98:1248–51. doi: 10.1093/jnci/djj335. [DOI] [PubMed] [Google Scholar]
- 407.Swindall AF, Bellis SL. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem. 2011;286:22982–90. [DOI] [PMC free article] [PubMed]
- 408.Rasper DM, Vaillancourt JP, Hadano S, Houtzager VM, Seiden I, Keen SLC, et al. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–88. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 409.Cardinale CJ, Wei Z, Panossian S, Wang F, Kim CE, Mentch FD, et al. Targeted resequencing identifies defective variants of decoy receptor 3 in pediatric-onset inflammatory bowel disease. Genes Immun. 2013;14:447–52. doi: 10.1038/gene.2013.43. [DOI] [PubMed] [Google Scholar]
- 410.Grossman I, Avidan N, Singer C, Goldstaub D, Hayardeny L, Eyal E, et al. Pharmacogenetics of glatiramer acetate therapy for multiple sclerosis reveals drug-response markers. Pharmacogenet Genom. 2007;17:657–66. doi: 10.1097/FPC.0b013e3281299169. [DOI] [PubMed] [Google Scholar]
- 411.Yolcu ES, Askenasy N, Singh NP, Cherradi S-EL, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 412.Legembre P, Micheau O, Ségui B. Chemotherapy with ceramide in TNBC. Oncoscience. 2015;2:817. doi: 10.18632/oncoscience.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Nahimana A, Aubry D, Lagopoulos L, Greaney P, Attinger A, Demotz S, et al. A novel potent Fas agonist for selective depletion of tumor cells in hematopoietic transplants. Blood Cancer J. 2011;1:e47. doi: 10.1038/bcj.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-α2b. Clin Cancer Res. 2007;13:2422–8. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 415.Tourneur L, Mistou S, Michiels F-M, Devauchelle VV, Renia L, Feunteun J, et al. Loss of FADD protein expression results in a biased Fas-signaling pathway and correlates with the development of tumoral status in thyroid follicular cells. Oncogene. 2003;22:2795–804. doi: 10.1038/sj.onc.1206399. [DOI] [PubMed] [Google Scholar]
- 416.McDermott U, Longley DB, Galligan L, Allen W, Wilson T, Johnston PG. Effect of p53 status and STAT1 on chemotherapy-induced, Fas-mediated apoptosis in colorectal cancer. Cancer Res. 2005;65:8951–60. doi: 10.1158/0008-5472.CAN-05-0961. [DOI] [PubMed] [Google Scholar]
- 417.Sudarshan S, Holman DH, Hyer ML, Voelkel-Johnson C, Dong JY, Norris JS. In vitro efficacy of Fas ligand gene therapy for the treatment of bladder cancer. Cancer Gene Ther. 2005;12:12–8. doi: 10.1038/sj.cgt.7700746. [DOI] [PubMed] [Google Scholar]
- 418.Eichhorst ST, Müller M, Li-Weber M, Schulze-Bergkamen H, Angel P, Krammer PH. A novel AP-1 Eelement in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol Cell Biol. 2000;20:7826–37. doi: 10.1128/mcb.20.20.7826-7837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA. 1997;94:13862. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Friesen C, Herr I, Krammer PH, Debatin K-M. Involvement of the CD95 (APO–1/Fas) receptor/ligand system in drug–induced apoptosis in leukemia cells. Nat Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 421.Wong GH, Goeddel DV. Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J Immunol. 1994;152:1751–5. [PubMed]
- 422.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–7. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 423.Zhu B, Luo L, Chen Y, Paty DW, Cynader MS. Intrathecal Fas ligand infusion strengthens immunoprivilege of central nervous system and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1561–9. doi: 10.4049/jimmunol.169.3.1561. [DOI] [PubMed] [Google Scholar]
- 424.Poulaki V, Mitsiades CS, McMullan C, Fanourakis G, Negri J, Goudopoulou A, et al. Human retinoblastoma cells are resistant to apoptosis induced by death receptors: role of caspase-8 gene silencing. Investig Ophthalmol Vis Sci. 2005;46:358–66. doi: 10.1167/iovs.04-0324. [DOI] [PubMed] [Google Scholar]
- 425.Ramiro L, Abraira L, Quintana M, García-Rodríguez P, Santamarina E, Álvarez-Sabín J, et al. Blood biomarkers to predict long-term mortality after ischemic stroke. Life. 2021;11:135. doi: 10.3390/life11020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Muthumani K, Choo AY, Hwang DS, Premkumar A, Dayes NS, Harris C, et al. HIV-1 Nef-induced FasL induction and bystander killing requires p38 MAPK activation. Blood. 2005;106:2059–68. doi: 10.1182/blood-2005-03-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Pira GL, Cecca SDI, Montanari M, Moretta L, Manca F. Specific removal of alloreactive T-cells to prevent GvHD in hemopoietic stem cell transplantation: rationale, strategies and perspectives. Blood Rev. 2016;30:297–307. doi: 10.1016/j.blre.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 428.Zacks DN, Zheng Q-D, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Investig Ophthalmol Vis Sci. 2004;45:4563–9. doi: 10.1167/iovs.04-0598. [DOI] [PubMed] [Google Scholar]
- 429.Popov SG, Villasmil R, Bernardi J, Grene E, Cardwell J, Wu A, et al. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–55. doi: 10.1016/S0006-291X(02)00227-9. [DOI] [PubMed] [Google Scholar]
- 430.Dufour F, Sasseville AM-J, Chabaud S, Massie B, Siegel RM, Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis Int J Program Cell Death. 2011;16:256–71. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- 431.Berndt C, Möpps B, Angermüller S, Gierschik P, Krammer PH. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc Natl Acad Sci. 1998;95:12556–61. doi: 10.1073/pnas.95.21.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Georgantas RW, Leong KW, August JT. Antigen-specific induction of peripheral T cell tolerance in vivo by codelivery of DNA vectors encoding antigen and Fas ligand. Hum Gene Ther. 2004;11:851–8. doi: 10.1089/10430340050015464. [DOI] [PubMed] [Google Scholar]
- 433.Nyhus JK, Wolford C, Feng L, Barbera-Guillem E. Direct in vivo transfection of antisense Fas-ligand reduces tumor growth and invasion. Gene Ther. 2001;8:209–14. doi: 10.1038/sj.gt.3301372. [DOI] [PubMed] [Google Scholar]
- 434.Zhang H, Taylor J, Luther D, Johnston J, Murray S, Wyatt JR, et al. Antisense oligonucleotide inhibition of Bcl-xL and Bid expression in liver regulates responses in a mouse model of Fas-induced fulminant hepatitis. J Pharm Exp Ther. 2003;307:24–33. doi: 10.1124/jpet.103.050435. [DOI] [PubMed] [Google Scholar]
- 435.Filion MC, Filion B, Roy J, Ménard S, Reader S, Phillips NC. Development of immunomodulatory six base-length non-CpG motif oligonucleotides for cancer vaccination. Vaccine. 2004;22:2480–8. doi: 10.1016/j.vaccine.2003.11.072. [DOI] [PubMed] [Google Scholar]
- 436.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 437.Hasegawa A, Cheng X, Kajino K, Berezov A, Murata K, Nakayama T, et al. Fas-disabling small exocyclic peptide mimetics limit apoptosis by an unexpected mechanism. Proc Natl Acad Sci. 2004;101:6599–604. doi: 10.1073/pnas.0401597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, et al. A mechanism of cell survival sequestration of Fas by the HGF receptor met. Mol Cell. 2002;9:411–21. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 439.Kang SM, Braat D, Schneider DB, O’Rourke RW, Lin Z, Ascher NL, et al. A non-cleavable mutant of Fas ligand does not prevent neutrophilic destruction of islet transplants. Transplantation. 2000;69:1813–7. doi: 10.1097/00007890-200005150-00014. [DOI] [PubMed] [Google Scholar]
- 440.Kotturi HSR, Li J, Branham-O’Connor M, Stickel SL, Yu X, Wagner TE, et al. Tumor cells expressing a fusion protein of MULT1 and Fas are rejected in vivo by apoptosis and NK cell activation. Gene Ther. 2008;15:1302–10. doi: 10.1038/gt.2008.77. [DOI] [PubMed] [Google Scholar]
- 441.Deng GM, Zheng L, Chan FKM, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–72. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 442.Sakka L, Delétage N, Lalloué F, Duval A, Chazal J, Lemaire J-J, et al. SCO-spondin derived peptide NX210 induces neuroprotection in vitro and promotes fiber regrowth and functional recovery after spinal cord injury. PLoS ONE. 2014;9:e93179. doi: 10.1371/journal.pone.0093179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Boisguérin P, Covinhes A, Gallot L, Barrère C, Vincent A, Busson M, et al. A novel therapeutic peptide targeting myocardial reperfusion injury. Cardiovasc Res. 2020;116:633–44. doi: 10.1093/cvr/cvz145. [DOI] [PubMed] [Google Scholar]
- 444.Slesarev VI, Ellithorpe R, Dimitroff T. Inhibition of systemic TNF-alpha cytotoxicity in cancer patients by D-peptidoglycan. Med Oncol. 1998;15:37–43. doi: 10.1007/BF02787343. [DOI] [PubMed] [Google Scholar]
- 445.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–9. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–51. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 447.Lasham A, Lindridge E, Rudert F, Onrust R, Watson J. Regulation of the human fas promoter by YB-1, Puralpha and AP-1 transcription factors. Gene. 2000;252:1–13. doi: 10.1016/s0378-1119(00)00220-1. [DOI] [PubMed] [Google Scholar]
- 448.Lasham A, Moloney S, Hale T, Homer C, Zhang YF, Murison JG, et al. The Y-box-binding protein, YB1, is a potential negative regulator of the p53 tumor suppressor. J Biol Chem. 2003;278:35516–23. doi: 10.1074/jbc.M303920200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and Supplementary Materials.